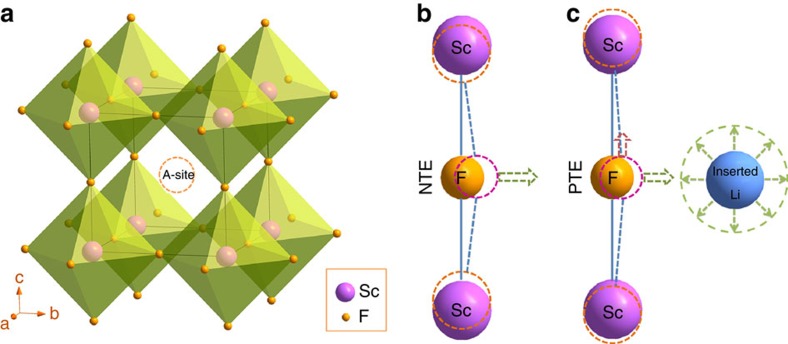
Figure 1. The effect of Li ion interaction on the tunable thermal expansion of ScF3.
(a) The cubic structure of ScF3 with open framework (space group: 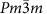 ). The cage consisting of corner-shared ScF3 regular octahedra is marked with the dash line circle (A-site). The guest ions or molecules can be inserted at the A-site cage. (b) The negative thermal expansion of ScF3 induced by the transverse vibration of fluorine normal to the linkage of Sc–F–Sc. (c) The steric hindrance role of inserted ions, eg, Li+, in the vibration of fluorine. The longitudinal vibration of fluorine results in the positive thermal expansion.
). The cage consisting of corner-shared ScF3 regular octahedra is marked with the dash line circle (A-site). The guest ions or molecules can be inserted at the A-site cage. (b) The negative thermal expansion of ScF3 induced by the transverse vibration of fluorine normal to the linkage of Sc–F–Sc. (c) The steric hindrance role of inserted ions, eg, Li+, in the vibration of fluorine. The longitudinal vibration of fluorine results in the positive thermal expansion.