Abstract
Background
Heart failure (HF) patient education aims to foster patients’ self-management skills. These are assumed to bring about, in turn, improvements in distal outcomes such as quality of life. The purpose of this study was to test the hypothesis that change in self-reported self-management skills observed after participation in self-management education predicts changes in physical and mental quality of life and depressive symptoms up to one year thereafter.
Methods
The sample comprised 342 patients with chronic heart failure, treated in inpatient rehabilitation clinics, who received a heart failure self-management education program. Latent change modelling was used to analyze relationships between both short-term (during inpatient rehabilitation) and intermediate-term (after six months) changes in self-reported self-management skills and both intermediate-term and long-term (after twelve months) changes in physical and mental quality of life and depressive symptoms.
Results
Short-term changes in self-reported self-management skills predicted intermediate-term changes in mental quality of life and long-term changes in physical quality of life. Intermediate-term changes in self-reported self-management skills predicted long-term changes in all outcomes.
Conclusions
These findings support the assumption that improvements in self-management skills may foster improvements in distal outcomes.
Keywords: Chronic heart failure, Self-management, Quality of life, Latent change, Cardiac rehabilitation, Patient education
Background
Chronic heart failure (HF) is a common, disabling and fatal medical condition [1]. In view of the aging population it will affect even more people in the future [2]. It requires multidisciplinary management programs including patient education concerning self-care/self-management [3–5]. Self-management includes management of symptoms, treatment, consequences and lifestyle changes implicated by HF and aims to maintain quality of life [6]. For patients with HF, self-monitoring and responding to changes in symptoms are central components of self-management. The situation-specific theory of heart failure self-care [7, 8] states that self-care is a decision-making process, which involves maintenance (treatment adherence and healthy behavior), symptom perception and management (response to symptoms). Self-management interventions proved to be effective with regard to knowledge, self-efficacy, self-management behavior, quality of life, hospitalization, and mortality [9–11]. Thus, self-management is considered the central outcome of such programs and a means to achieve other important outcomes [5, 12]. Self-management can be conceived of as an important proximal outcome of patient education that is a necessary (but not sufficient) prerequisite for the achievement of more distal goals as course of disease, quality of life or social participation [13]. Furthermore, in addition to improving self-management skills during treatment, it is important for patients to sustain and apply the acquired skills after treatment.
Studies have shown that quality of life in HF patients is reduced compared to the general population or patients with other chronic conditions [14–16]. Furthermore, depression is common among patients with HF and worsens the prognosis [17–19]. Therefore, both quality of life and depressive symptoms are considered important distal outcomes in patients with HF.
However, the relations between proximal and distal outcomes of self-management programs have rarely been studied so far. For example, there is no evidence yet that in patients with HF improvement in self-management is actually associated with subsequent improvement in quality of life. Most studies only report separate effects on different proximal and distal outcomes without relating them to each other [10, 11, 20], e. g., without examining whether improvements in knowledge or self-care lead to improvements in quality of life. Two cross-sectional studies showed that self-care behavior (in contrast to self-care confidence) was not associated with higher quality of life in patients with HF [21, 22]. However, these studies did not take into account changes in self-care or quality of life. According to the situation-specific theory of heart failure self-care [7, 8], one study tested whether there were different patterns of change in HF self-care management and whether these were associated with different patterns of change in quality of life over six months [23]. Results show that those who improved in self-care management over time also improved in quality of life. However, the study did not include an intervention and investigated concurrent rather than subsequent changes in outcome variables. In a previous study in patients with different chronic disorders, we showed that short-term changes (before/after inpatient rehabilitation including patient education) in self-reported self-management skills predicted 3-months changes in quality of life and depressive symptoms [24]. The purpose of the present study was to examine whether similar relationships are found in patients with HF and to extend the follow-up period. Particularly, this study investigated whether patients with HF who report an increase in self-reported self-management skills both directly after inpatient rehabilitation including self-management education and after six months show a subsequent increase in quality of life and a decrease in depressive symptoms after both six and twelve months. We hypothesized (1) that the difference of self-reported self-management skills between start and end of rehabilitation (duration 3 weeks) predicts the difference of both quality of life and depressive symptoms between start of rehabilitation and follow-up measurements six and twelve months later. We further hypothesized (2) that the difference of self-reported self-management skills between start of rehabilitation and follow-up after six months predicts the difference of both quality of life and depressive symptoms between start of rehabilitation and follow-up after twelve months.
Methods
Participants and procedure
This is a secondary analysis based on data from a study that evaluated a self-management patient education program for patients with chronic heart failure in inpatient cardiac rehabilitation [25] the results of which were presented elsewhere [26]. A cluster-randomized trial of patients with HF was conducted in four rehabilitation clinics. All participants received a 3-week multidisciplinary inpatient rehabilitation including medical treatment, exercise therapy/physical training, health education, psychological support, relaxation and social counselling as well as a self-management patient education program of different lengths: Patients in the intervention group received a new self-management educational program consisting of five interactive sessions of at least 60 min each provided in small groups. A physician, a nurse, a psychologist and a physiotherapist, respectively, held the interdisciplinary program. Contents were disease and treatment knowledge, self-management behaviors, medication, promotion of physical activity, illness related problems in everyday life, emotional distress and coping strategies. Patients in the control group received a short lecture-based educational program (one lecture of 60 min held by a physician). Contents were disease information and self-management recommendations.
Patients were consecutively included if they had a diagnosis of chronic systolic heart failure (ICD-10: I50) with left ventricular ejection fraction (LVEF) of ≤ 40 and New York Heart Association (NYHA) functional classification II or III. Exclusion criteria were acute events of decompensation, cognitive impairment, insufficient German language ability and severe visual or hearing impairment. Patients completed several standardized patient reported questionnaires at the start (T1) and end (T2) of inpatient rehabilitation as well as after six (T3) and twelve months (T4). Participation in the study was voluntary and all participants provided written informed consent. The study complied with the Declaration of Helsinki and was approved by the ethical review committee of the Faculty of Medicine, University of Würzburg (reference number: 60/11). Detailed information about this trial is presented elsewhere [26]. Of 517 eligible patients 475 comprised the initial study sample. The present analyses are based on those n = 342 patients (72%) with data at all four measurement points.
The data of both intervention group (n = 178) and control group (n = 164) patients were analyzed together, because all received a patient self-management education program. This study does not investigate differences between different education programs but relationships in changes after education programs.
Measures
We used latent constructs, with selected items from standardized questionnaires serving as indicators.
Self-management skills were assessed with the Skill and technique acquisition subscale of the Health Education Impact Questionnaire (heiQTM) [27, 28]. It asks for the subjective appraisal of skills and techniques that help manage a chronic disease and related problems (item example: “When I have symptoms, I have skills that help me cope”). It proved to be reliable (Raykov’s Composite Reliability Coefficient = 0.77), uni-dimensional and measurement invariant over time [27, 29]. The items are assessed on a 1-to-4 point Likert scale with higher scores indicating higher skills. All 4 items were used as single indicators for the latent construct Self-management skills.
Quality of life was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) [30, 31], a disease-specific quality of life measure which proved to be reliable, valid and sensitive to clinical change. The 3 items of the subscale (mental) quality of life (item example: “Over the past 2 weeks, how much has your heart failure limited your enjoyment of life?”) were used as indicators of the latent construct Mental quality of life. For the latent construct Physical quality of life, 3 (out of 6) items of the physical limitation subscale representing moderate physical activity were chosen as indicators. This subscale measures impairment of everyday activities by HF and thus captures an important aspect of physical quality of life. An item example is: “Please indicate how much you are limited by heart failure (shortness of breath or fatigue) in your ability to do the following activities over the past 2 weeks: Climbing a flight of stairs without stopping”. We did not chose the items covering light physical activity (e.g., dressing or bathing) because we supposed that they might be too “easy” for this sample. All items are scaled from 1 to 5, with higher scores indicating a higher level of quality of life. For the items covering Physical quality of life, the response format included a possibility to indicate that the activity was limited or not done for other reasons. Due to this, the sample for the analyses concerning Physical quality of life was limited to those n = 214 patients who did not indicate this response option in one of these items at any measurement point. The patients who were excluded did not differ from the remaining patients with respect to age, sex, living with a partner, educational level, working status, New York Heart Association (NYHA) class and mean left ventricular ejection fraction (LVEF), respectively (data not shown).
Depressive symptoms were assessed with the 2-item-version of the depression module of the Patient Health Questionnaire, PHQ-2 [32]. This reliable (α = 0.83) and valid measure contains two main criteria of depression, diminished interest or pleasure and depressed mood (“Over the past 2 weeks, how often have you been bothered by any of the following problems? Little interest or pleasure in doing things”; “Feeling down, depressed or hopeless”). Items are scaled from 1 to 4, with higher scores indicating higher levels of depressive symptoms. Both items were used as single indicators of the latent construct Depressive symptoms.
Statistical analyses
Statistical analyses were performed similar to Musekamp et al. [24], and detailed description of analytical procedures can be found there. Structural equation modeling techniques were used to investigate the hypotheses.
All models were computed using Mplus version 7.11 [33]. Full information maximum likelihood (FIML) algorithm [34] was used to handle missing data. Proportion of missing data was low (≤7%) for all variables. All models were estimated using a maximum likelihood estimator with robust standard errors [35]. Model fit was assessed based on Chi-square goodness of fit test, Comparative Fit Index (CFI) and Root Mean Square Error of Approximation (RMSEA), with CFI close to or higher than 0.95 and RMSEA close to or lower than 0.06 indicating good model fit [36]. Alpha was set to 0.05 for all analyses.
As preliminary analyses, confirmatory factor analyses (CFA) were applied to test measurement invariance (scalar invariance) over time [37, 38].
Then, to examine the hypotheses, changes in self-reported Self-management skills and changes in Quality of life and Depressive symptoms were modelled applying Latent True Change Modeling [39–42]. This approach allows modelling interindividual differences in intraindividual change in a structural equation framework. Thus, latent (“true”) change can be analysed without measurement error. For judging the size of changes, standardized effect sizes (SES = Mean/SD1) were computed for all latent change variables. Standardized estimates for path coefficients are reported for assessment of relations among latent variables. Separate models for the three outcomes (Physical quality of life, Mental quality of life, Depressive symptoms) were tested. For identifying the models, the loading of the first item of each factor was fixed to 1, and the first intercept of each factor was fixed to 0. To model indicator-specific effects, correlations between the same indicators (items) at different measurement points were allowed [43]. Two types of models were estimated for all outcomes. The models of the first type use all four measurement points. They predict that changes in self-reported Self-management skills between T1 and T2 predict changes in Physical quality of life (model A1), Mental quality of life (model B1) and Depressive symptoms (model C1) between T1 and T3 and between T1 and T4, respectively (subsequent changes). They further assume the prediction of changes in these outcomes between T1 and T2 (concurrent changes). The models of the second type use the measurement points T1, T3 and T4. These models predict that changes in self-reported Self-management skills between T1 and T3 predict changes in the three outcomes (Physical quality of life: model A2, Mental quality of life: model B2, Depressive symptoms: model C2) between T1 and T4 (subsequent changes), in addition to the prediction of changes between T1 and T3 (concurrent changes).
Results
Sample
The sample comprises 342 patients with HF. Table 1 presents the sociodemographic and clinical characteristics. Mean age was 62.0 years (SD = 10.7), 76% were male, and 71% were living with a partner. Educational level was vocational training for most (68%). Most participants were retired (43%) or employed (42%). New York Heart Association (NYHA) class was II for 58%, mean left ventricular ejection fraction (LVEF) was 32.3 (SD = 6.7).
Table 1.
Sample characteristics (n = 342)
| n | % | |
| Male sex | 261 | 76,3 |
| Living with a partnera | 244 | 71,3 |
| Educational levelb | ||
| Vocational training | 232 | 67,8 |
| Technical college | 38 | 11,1 |
| University | 19 | 5,6 |
| Other | 40 | 11,7 |
| Working statusc | ||
| Employed | 143 | 41,8 |
| Retirement | 147 | 43,0 |
| Unemployed | 27 | 7,9 |
| Other | 24 | 7,0 |
| NYHA IId | 197 | 57,6 |
| M | SD | |
| Age | 62,0 | 10,7 |
| LVEFc | 32,3 | 6,7 |
NYHA New York Heart Association class, LVEF left ventricular ejection fraction;a missing: n = 3.b missing: n = 13.c missing: n = 1.d missing: n = 7
Preliminary analyses
CFAs testing for configural invariance over time showed good model fit for all variables (CFI > 0.99, RMSEA < 0.05). Metric and scalar invariance models still showed good model fit for all variables (CFI > 0.96, RMSEA < 0.08). There were no misspecifications of parameters as shown by EPC and modification indices. Thus, the assumption of scalar measurement invariance over time was met.
Latent true change models
Model fit
All latent change models yielded good fit with CFI > 0.96 and RMSEA > 0.05 (Table 2). Although the Chi-quare test was significant in some cases, the other fit indices indicated a good approximate fit of the models.
Table 2.
Goodness of fit summary for latent change models
| Outcome | χ 2 | df | p | CFI | RMSEA |
|---|---|---|---|---|---|
| Model A1: Physical quality of life | |||||
| (T1 throughT4) | 174.40 | 151 | 0.093 | 0.99 | 0.03 |
| Model B1: Mental quality of life | |||||
| (T1 throughT4) | 263.44 | 151 | <0.001 | 0.97 | 0.05 |
| Model C1: Depressive symptoms | |||||
| (T1 throughT4) | 98.24 | 85 | 0.155 | 0.99 | 0.02 |
| Model A2: Physical quality of life | |||||
| (T1, T3, T4) | 118.10 | 110 | 0.282 | 1.00 | 0.02 |
| Model B2: Mental quality of life | |||||
| (T1, T3, T4) | 150.59 | 110 | 0.006 | 0.99 | 0.03 |
| Model C2: Depressive symptoms | |||||
| (T1, T3, T4) | 82.61 | 67 | 0.095 | 0.99 | 0.03 |
CFI comparative fit index, RMSEA root mean square error of approximation
Model parameters and effect sizes
Table 3 shows the unstandardized intercepts and factor loadings for the six latent change models. All indicators of one factor have comparable factor loadings, are thus rather homogenous and contribute similarly to the latent constructs.
Table 3.
Estimated intercepts and unstandardized factor loadings for the latent change models
| Model | 1: measurement points T1 through T4 | 2: measurement points T1, T3, T4 | |||
|---|---|---|---|---|---|
| Item | Intercept | Unstandardized factor loading (SE) | Intercept | Unstandardized factor loading (SE) | |
| A | SM1 | 0.00* | 1.00* (−) | 0.00* | 1.00* (−) |
| SM2 | −0.12 | 1.13 (0.09) | −0.07 | 1.09 (0.09) | |
| SM3 | −0.19 | 1.16 (0.10) | 0.01 | 1.07 (0.09) | |
| SM4 | 0.82 | 0.89 (0.09) | 1.00 | 0.81 (0.08) | |
| pQL1 | 0.00* | 1.00* (−) | 0.00* | 1.00* (−) | |
| pQL2 | −0.37 | 1.08 (0.04) | −0.37 | 1.08 (0.05) | |
| pQL3 | −0.93 | 1.06 (0.05) | −0.89 | 1.06 (0.05) | |
| B | SM1 | 0.00* | 1.00* (−) | 0.00* | 1.00* (−) |
| SM2 | −0.23 | 1.16 (0.06) | −0.20 | 1.14 (0.06) | |
| SM3 | −0.11 | 1.11 (0.07) | −0.15 | 1.12 (0.07) | |
| SM4 | 0.97 | 0.83 (0.06) | 0.89 | 0.84 (0.06) | |
| mQL1 | 0.00* | 1.00* (−) | 0.00* | 1.00* (−) | |
| mQL2 | −0.41 | 0.96 (0.04) | −0.43 | 0.98 (0.04) | |
| mQL3 | 0.46 | 0.89 (0.03) | 0.49 | 0.89 (0.03) | |
| C | SM1 | 0.00* | 1.00* (−) | 0.00* | 1.00* (−) |
| SM2 | −0.26 | 1.17 (0.06) | −0.22 | 1.14 (0.06) | |
| SM3 | −0.15 | 1.13 (0.07) | −0.16 | 1.12 (0.07) | |
| SM4 | 0.94 | 0.84 (0.06) | 0.90 | 0.84 (0.06) | |
| D1 | 0.00* | 1.00* (−) | 0.00* | 1.00* (−) | |
| D2 | −0.04 | 0.83 (0.09) | −0.06 | 0.85 (0.06) | |
Fixed parameters are marked with an asterisk. Intercepts and unstandardized factor loadings of same indicators were set equal over measurement points. SM1 to SM4 = Indicators for Self-management skills, pQL1 to pQL3 = Indicators for Physical quality of life, mQL1 to mQL4 = Indicators for Mental quality of life, D1 and D2 = Indicators for Depressive symptoms. SE = standard error
The means, variances and effect sizes (standardized effect size, SES) of latent change variables for all models are shown in table 4. All means of all latent change variables were significantly different from zero, which indicates change in these variables over measurement points. In addition, all variances of all latent change variables were significantly different from zero, indicating interindividual differences in intraindividual change. Effect sizes show that changes in Self-management skills are of medium size, changes in Physical quality of life and Depressive symptoms are small to medium and changes in Mental quality of life are medium to large.
Table 4.
Unstandardized means and variances and effect sizes of latent change variables
| LCV | Model | Mean | Variance | SES |
|---|---|---|---|---|
| ∆SM2 − SM1 | A1 | 0.26* | 0.23* | 0.48 |
| B1, C1 | 0.29* | 0.25* | 0.50 | |
| ∆SM3 − SM1 | A2 | 0.27* | 0.28* | 0.47 |
| B2, C2 | 0.31* | 0.26* | 0.53 | |
| ∆pQL2 − pQL1 | A1 | 0.23* | 0.80* | 0.23 |
| ∆pQL3 − pQL1 | A1 | 0.36* | 0.97* | 0.37 |
| A2 | 0.35* | 0.96* | 0.36 | |
| ∆pQL4 − pQL1 | A1 | 0.37* | 0.91* | 0.37 |
| A2 | 0.37* | 0.91* | 0.37 | |
| ∆mQL2 − mQL1 | B1 | 0.52* | 0.31* | 0.58 |
| ∆mQL3 − mQL1 | B1 | 0.63* | 0.73* | 0.71 |
| B2 | 0.65* | 0.73* | 0.72 | |
| ∆mQL4 − mQL1 | B1 | 0.71* | 0.78* | 0.80 |
| B2 | 0.73* | 0.78* | 0.81 | |
| ∆D2 − D1 | C1 | −0.29* | 0.24* | −0.38 |
| ∆D3 − D1 | C1 | −0.28* | 0.49* | −0.36 |
| C2 | −0.28* | 0.50* | −0.35 | |
| ∆D4 − D1 | C1 | −0.30* | 0.61* | −0.38 |
| C2 | −0.30* | 0.61* | −0.38 |
LCV ∆ = latent change variable, SM Self-management skills, pQL Physical quality of life, mQL Mental quality of life, D Depressive symptoms, SES Standardized effect size. Subscripted figures represent measurement points
* p < .05
Path coefficients
Figure 1 shows the standardized path coefficients for the structural models A1, B1 and C1 with measurement points T1 through T4. In model A1, interindividual differences in intraindividual change in Physical quality of life between T1 and T4 were significantly predicted by change in Self-management skills between T1 and T2 in such a way that increases of Self-management skills predicted increases in Physical quality of life (β = 0.23). Changes in Self-management skills did not predict changes in Physical quality of life between T1 and T2 or T1 and T3, respectively. In model B1, increases in Self-management skills predicted increases in Mental quality of life between T1 and T2 (β = 0.36) and between T1 and T3 (β = 0.24), respectively, but not between T1 and T4. In model C1, increases in Self-management skills predicted decreases in Depressive symptoms between T1 and T2 (β = −0.29), but not at the later occasions. Additionally, the baseline level of Self-management skills predicted changes in both Mental quality of life and Depressive Symptoms between T1 and T4.
Fig. 1.
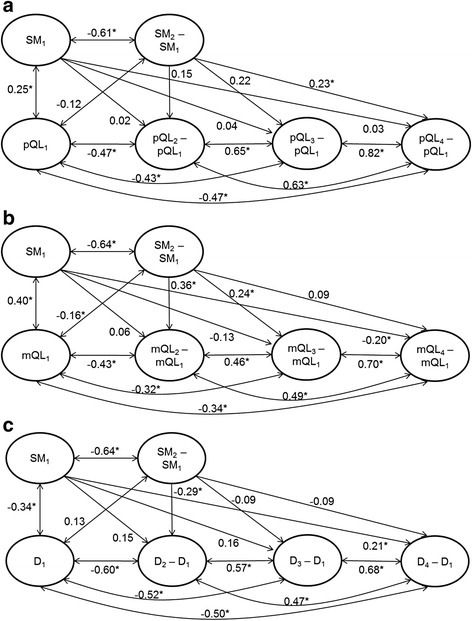
Latent change model (structural model) with standardized path coefficients. Predictor Self-management skills (SM), measurement points T1 throughT4. A1: Outcome Physical quality of life (pQL). B1: Outcome Mental quality of life (mQL). C1: Outcome Depressive symptoms (D). Circles represent latent variables, single-headed arrows show the impact of one variable on another, double arrows show correlations allowed between variables. Subscripted figures represent measurement points. *p < 0.05
The standardized path coefficients in models A2, B2 and C2 with measurement points T1, T3 and T4 are shown in Fig. 2. In all three models, changes in Self-management skills between T1 and T3 predict changes in distal outcomes between T1 and T4. Increases in Self-management skills predict increases in Physical quality of life (β = 0.39), increases in Mental quality of life (β = 0.22), and decreases in Depressive symptoms (β = −0.20). Additionally, changes between T1 and T3 in all three outcomes were also predicted by changes in Self-management skills (β = 0.32, β = 0.38 and β = −0.34, respectively). Furthermore, the baseline level of Self-management skills predicted changes in Depressive symptoms between T1 and T4.
Fig. 2.
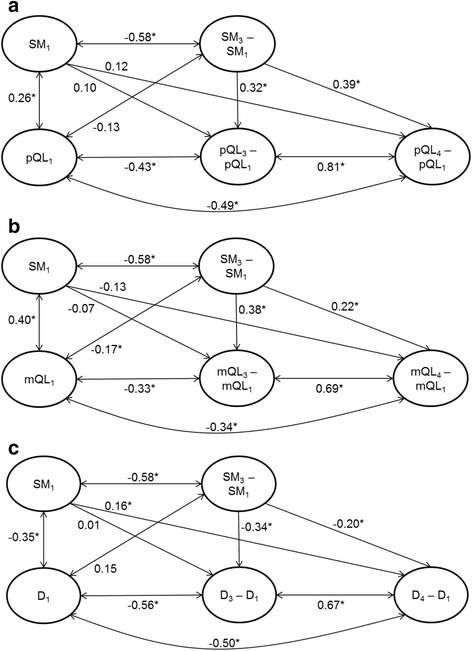
Latent change model (structural model) with standardized path coefficients. Predictor Self-management skills (SM), measurement points T1, T3, and T4. A2: Outcome Physical quality of life (pQL). B2: Outcome Mental quality of life (mQL). C2: Outcome Depressive symptoms (D). Circles represent latent variables, single-headed arrows show the impact of one variable on another, double arrows show correlations allowed between variables. Subscripted figures represent measurement points. *p < 0.05
Discussion
To our knowledge, this is the first study that examined the associations between change in self-reported self-management skills and subsequent change in quality of life and depressive symptoms in patients with HF undergoing inpatient rehabilitation including self-management patient education. Results show that changes in self-management skills predict changes in these distal outcomes. Concerning hypothesis 1, results differ between outcomes. Short-term change in self-reported self-management skills (between start and end of rehabilitation) predicted long-term change in physical quality of life (between start of rehabilitation and follow-up twelve months later), but not intermediate-term change (after six months). By contrast, it predicted intermediate-term, but not long-term, change in mental quality of life. Finally, it did neither predict intermediate nor long-term change in depressive symptoms. Thus, hypothesis 1 was only partly confirmed. Regarding hypothesis 2, the picture is consistent: Intermediate-term change in self-reported self-management skills (between start of rehabilitation and follow-up after six months) predicted long-term changes in all three outcomes (between start of rehabilitation and follow-up after twelve months). Thus, hypothesis 2 was confirmed.
The results are in line with Musekamp et al. [24], but not only confirm the prior findings in a HF sample but also expand them with regard to the follow-up period. Although there may be differences in concrete self-management activities between different conditions, increase in self-reported self-management skills is associated with subsequent increase in quality of life and decrease in depressive symptoms regardless of condition. These results are consistent with definitions of self-management and models of patient education in patients with chronic conditions in general suggesting such relationships between self-management skills and quality of life [6, 13]. They are also consistent with the propositions of the situation specific theory of heart failure self-care [8]. Together, they support the importance of self-management in HF patient education [4, 5].
Our results suggest that, in patients with HF, the acquirement of self-management skills during self-management education may have a long-term influence on physical quality of life and an intermediate-term influence on mental quality of life. Thus, improved self-management skills may influence mental quality of life more directly, because they immediately convey a sense of exerting control. Physical quality of life may be influenced in a delayed (but more sustainable) fashion, however, because it takes more time to influence physical conditions. For example, it takes some time until the appropriate reaction to HF symptoms affects the physical condition and the individual takes notice of this improvement. For the long-term changes in mental quality of life and depressive symptoms the baseline level of self-management skills seems a better predictor than the short-term change in self-management skills.
Changes in self-reported self-management skills were not only observed immediately after rehabilitation, but also after six months, with medium effect sizes. Thus, the aim of rehabilitation and self-management patient education to sustain self-management skills in everyday life was reached. Interestingly, change of self-management skills up to six months after rehabilitation seems to influence further change in distal outcomes. Thus, it is not only important to increase self-management skills during self-management education, but also to sustain them afterwards. This emphasizes the importance of fostering self-management skills that can be applied and sustained after treatment. Self-management programs should therefore have a strong focus on everyday life and implement aftercare plans.
However, it remains unclear, whether changes in distal outcomes are initiated by self-management education alone or also by other processes or events coming into effect during the follow-up period. This might for example include events like job changes or retirement.
Further studies should investigate treatment mechanisms of self-management patient education, as they are still unclear [44]. The causal pathway between self-management skills and quality of life needs further examination. It should be examined whether the influence of self-management skills is mediated by actual self-management/self-care behaviors, such as symptom monitoring and responding to symptoms or health behaviors, such as adherence to medication or moderate physical activity [4, 8]. These variables should be included in future models to clarify the effects of self-management skills and other potential predictor variables. Others [45] have investigated the effect of improvement in knowledge after a nurse-led education session on clinical outcomes and found associations with reduced hospital readmissions. On the other hand, knowledge alone may not be sufficient to establish adequate self-care [46]. Therefore, it should be investigated how knowledge and skills work together in establishing adequate self-management behavior and quality of life.
Limitations
There are some limitations that should be considered. First, while longitudinal associations were examined, causality cannot be proved due to the non-experimental design of our study.
Second, the scale used to assess self-management skills is generic in nature and possibly does not cover all self-management skills important for patients with HF as symptom-monitoring [3]. Thus, our scale might not be specific enough to cover more than general expectations about self-management. Further studies should explore whether specific measures of self-management skills in HF add to prediction of distal outcomes. Third, it was based on self-report and thus susceptible to bias. However, it is difficult to implement objective measures of self-management. Fourth, possibly confounding factors influencing the course of both quality of life and depressive symptoms after self-management education were not taken into account in this study. For example, trait factors like dispositional optimism might influence assessment of both self-management skills and quality of life [47].
Conclusion
This study adds to previous evidence in that it supports the assumption that self-management skills improved during short- and intermediate-term are associated with intermediate and long-term improvements in both quality of life and depressive symptoms in patients with HF. Thus, relationships between an important proximal outcome of self-management patient education and various important distal outcomes could be demonstrated. The results support the conclusion that patient education interventions for patients with HF should target self-management skills. These should be addressed with a focus on sustainability and suitability in everyday life as some changes became evident only several months after the completion of the educational program. Further studies should investigate additional predictor variables as self-care behavior as well as treatment mechanisms of self-management programs.
Acknowledgements
The authors wish to thank the participating rehabilitation clinics: Rehabilitation Hospital Kirchberg-Klinik (Dr. Ernst Knoglinger), Rehabilitation Hospital Möhnesee (Dr. Rainer Schubmann), Rehabilitation Hospital Wetterau (Dr. Ulrich Kiwus), Segeberger Kliniken GmbH, Rehabilitation Hospital (Dr. Ronja Westphal), which supported the evaluation of the program.
Funding
This study was funded by the German Statutory Pension Insurance Scheme (Deutsche Rentenversicherung Bund; grant number: 8011 – 106 – 31/31.93.1). This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
Availability of data and materials
The dataset supporting the conclusion of this article is available on reasonable request from the corresponding author.
Authors’ contributions
GM is the principal investigator, developed the research question, undertook guidance of data collection, performed the statistical analyses and is the main author of the manuscript. MS gave statistical advice, contributed to the design of the study and drafted the manuscript. BS undertook guidance of data collection and helped draft the manuscript. JB and HF contributed to the design of the study and helped draft the manuscript. KM developed the primary study, contributed to the design of the study and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the ethical review committee of the Faculty of Medicine, University of Würzburg (reference number: 60/11).
Abbreviations
- CFA
Confirmatory factor analysis
- CFI
Comparative fit index
- D
Depressive symptoms
- FIML
Full information maximum likelihood
- heiQ
Health education impact questionnaire
- HF
Heart failure
- KCCQ
Kansas city cardiomyopathy questionnaire
- LVEF
Left ventricular ejection fraction
- M
Mean
- mQL
Mental quality of life
- NYHA
New York Heart Association
- PHQ-2
Patient health questionnaire depression module 2-item version
- pQL
Physical quality of life
- RMSEA
Root mean square error of approximation
- SD
Standard deviation
- SE
Standard error
- SES
Standardized effect size
- SM
Self-management skills
Contributor Information
Gunda Musekamp, Phone: +49 931 31 81056, Email: gunda.musekamp@uni-wuerzburg.de.
Michael Schuler, Email: m.schuler@uni-wuerzburg.de.
Bettina Seekatz, Email: b.seekatz@uni-wuerzburg.de.
Jürgen Bengel, Email: juergen.bengel@psychologie.uni-freiburg.de.
Hermann Faller, Email: h.faller@uni-wuerzburg.de.
Karin Meng, Email: k.meng@uni-wuerzburg.de.
References
- 1.McMurray JJV, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–89. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, MacIntyre K, Capewell S, McMurray JJ. Heart failure and the aging population: an increasing burden in the 21st century? Heart. 2003;89:49–53. doi: 10.1136/heart.89.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs033. [DOI] [PubMed] [Google Scholar]
- 4.Lainscak M, Blue L, Clark AL, Dahlström U, Dickstein K, Ekman I, et al. Self-care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:115–26. doi: 10.1093/eurjhf/hfq219. [DOI] [PubMed] [Google Scholar]
- 5.Strömberg A. The crucial role of patient education in heart failure. Eur J Heart Fail. 2005;7:363–9. doi: 10.1016/j.ejheart.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177–87. doi: 10.1016/S0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 7.Riegel B, Dickson VV. A situation-specific theory of heart failure self-care. J Cardiovasc Nurs. 2008;23:190–6. doi: 10.1097/01.JCN.0000305091.35259.85. [DOI] [PubMed] [Google Scholar]
- 8.Riegel B, Dickson VV, Faulkner KM. The situation-specific theory of heart failure self-care: revised and updated. J Cardiovasc Nurs. 2015 doi: 10.1097/JCN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 9.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78:297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnason S, Zimmerman L, Young L. An integrative review of interventions promoting self-care of patients with heart failure. J Clin Nurs. 2012;21:448–75. doi: 10.1111/j.1365-2702.2011.03907.x. [DOI] [PubMed] [Google Scholar]
- 12.Deaton C. Outcomes measurement. J Cardiovasc Nurs. 2000;14:116–8. doi: 10.1097/00005082-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Faller H, Reusch A, Meng K. DGRW-Update: Patiententschulung. Rehabilitation. 2011;50:284–91. doi: 10.1055/s-0031-1285889. [DOI] [PubMed] [Google Scholar]
- 14.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–41. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867–76. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 16.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–51. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, et al. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–8. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Faller H, Störk S, Schowalter M, Steinbüchel T, Wollner V, Ertl G, et al. Depression and survival in chronic heart failure: does gender play a role? Eur J Heart Fail. 2007;9:1018–23. [DOI] [PubMed]
- 20.Grady KL. Self-care and quality of life outcomes in heart failure patients. J Cardiovasc Nurs. 2008;23:285–92. doi: 10.1097/01.JCN.0000305092.42882.ad. [DOI] [PubMed] [Google Scholar]
- 21.Buck HG, Lee CS, Moser DK, Albert NM, Lennie T, Bentley B, et al. Relationship between self-care and health-related quality of life in older adults with moderate to advanced heart failure. J Cardiovasc Nurs. 2012;27:8–15. doi: 10.1097/JCN.0b013e3182106299. [DOI] [PubMed] [Google Scholar]
- 22.Seto E, Leonard KJ, Cafazzo JA, Masino C, Barnsley J, Ross HJ. Self-care and quality of life of heart failure patients at a multidisciplinary heart function clinic. J Cardiovasc Nurs. 2011;26:377–85. doi: 10.1097/JCN.0b013e31820612b8. [DOI] [PubMed] [Google Scholar]
- 23.Lee CS, Mudd JO, Hiatt SO, Gelow JM, Chien C, Riegel B. Trajectories of heart failure self-care management and changes in quality of life. Eur J Cardiovasc Nurs. 2015;14:486–94. doi: 10.1177/1474515114541730. [DOI] [PubMed] [Google Scholar]
- 24.Musekamp G, Schuler M, Bengel J, Faller H. Improved self-management skills predict improvements in quality of life and depression in patients with chronic disorders. Patient Educ Couns. 2016;99:1355–1361. doi:10.1016/j.pec.2016.03.022. [DOI] [PubMed]
- 25.Meng K, Musekamp G, Seekatz B, Glatz J, Karger G, Kiwus U, et al. Evaluation of a self-management patient education program for patients with chronic heart failure undergoing inpatient cardiac rehabilitation: study protocol of a cluster randomized controlled trial. BMC Cardiovasc Disord. 2013;13:60. doi: 10.1186/1471-2261-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng K, Musekamp G, Schuler M, Seekatz B, Glatz J, Karger G, et al. The impact of a self-management patient education program for patients with chronic heart failure undergoing inpatient cardiac rehabilitation. Patient Educ Couns. 2016;99:1190–1197. doi:10.1016/j.pec.2016.02.010. [DOI] [PubMed]
- 27.Schuler M, Musekamp G, Faller H, Ehlebracht-König I, Gutenbrunner C, Kirchhof R, et al. Assessment of proximal outcomes of self-management programs: translation and psychometric evaluation of a German version of the Health Education Impact Questionnaire (heiQTM) Qual Life Res. 2013;22:1391–403. doi: 10.1007/s11136-012-0268-6. [DOI] [PubMed] [Google Scholar]
- 28.Osborne RH, Elsworth GR, Whitfield K. The Health Education Impact Questionnaire (heiQ): An outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns. 2007;66:192–201. doi: 10.1016/j.pec.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Schuler M, Musekamp G, Bengel J, Schwarze M, Spanier K, Gutenbrunner C, et al. Measurement of stable changes of self-management skills after rehabilitation: a latent state–trait analysis of the Health Education Impact Questionnaire (heiQTM) Qual Life Res. 2014;23:2531–43. doi: 10.1007/s11136-014-0693-9. [DOI] [PubMed] [Google Scholar]
- 30.Faller H, Steinbüchel T, Schowalter M, Spertus JA, Störk S, Angermann CE. Der Kansas City Cardiomyopathy Questionnaire (KCCQ) - ein neues krankheitsspezifisches Messinstrument zur Erfassung der Lebensqualität bei chronischer Herzinsuffizienz. Psychometrische Prüfung der deutschen Version. Psychoth, Psychosom Med Psychol. 2005;55:200–8. doi: 10.1055/s-2004-834597. [DOI] [PubMed] [Google Scholar]
- 31.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/S0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 32.Löwe B, Kroenke K, Gräfe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J Psychosom Res. 2005;58:163–71. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Muthén LK, Muthén BO. Mplus user’s guide. 6. Muthén & Muthén: Los Angeles, CA; 2010. [Google Scholar]
- 34.Enders CK. The impcat of nonnormality on full information maximum-likelihood estimation for structural equation models with missing data. Psychol Methods. 2001;6:352–70. doi: 10.1037/1082-989X.6.4.352. [DOI] [PubMed] [Google Scholar]
- 35.Weston R, Gore PA. A brief guide to structural equation modeling. Couns Psychol. 2006;34:719–51. doi: 10.1177/0011000006286345. [DOI] [Google Scholar]
- 36.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 37.Millsap RE. Statistical approaches to measurement invariance. New York: Psychology Press; 2011. [Google Scholar]
- 38.Meredith W. Measurement invariance, factor analysis and factioral invariance. Psychom. 1993;58:525–43. doi: 10.1007/BF02294825. [DOI] [Google Scholar]
- 39.Geiser C, Eid M, Nussbeck FW, Courvoisier DS, Cole DA. Analyzing true change in longitudinal multitrait-multimethod-studies: application of a multimethod change model to depression and anxiety in children. Dev Psychol. 2010;46:29–45. doi: 10.1037/a0017888. [DOI] [PubMed] [Google Scholar]
- 40.Reuter T, Ziegelmann JP, Wiedemann AU, Geiser C, Lippke S, Schüz B, et al. Changes in intentions, planning, and self-efficacy predict changes in behaviors. J Health Psychol. 2010;15:935–47. doi: 10.1177/1359105309360071. [DOI] [PubMed] [Google Scholar]
- 41.Steyer R, Eid M, Schwenkmezger P. Modeling true intraindividual change: true change as a latent variable. Methods Psychol Res Online. 1997;2:21–33. [Google Scholar]
- 42.McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- 43.Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112:558–77. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- 44.Jonkman NH, Schuurmans MJ, Groenwold RH, Hoes AW, Trappenburg JC. Identifying components of self-management interventions that improve health-related quality of life in chronically ill patients: Systematic review and meta-regression analysis. Patient Educ Couns. 2016;99:1087–1098. doi:10.1016/j.pec.2016.01.022. [DOI] [PubMed]
- 45.Kommuri NVA, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86:233–8. doi: 10.1016/j.pec.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Dickson VV, Riegel B. Are we teaching what patients need to know? Building skills in heart failure self-care. Heart Lung. 2009;38:253–61. doi: 10.1016/j.hrtlng.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Kostka T, Jachimowicz V. Relationship of quality of life to dispositional optimism, health locus of control and self-efficacy in older subjects living in different environments. Qual Life Res. 2010;19:351–61. doi: 10.1007/s11136-010-9601-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusion of this article is available on reasonable request from the corresponding author.


