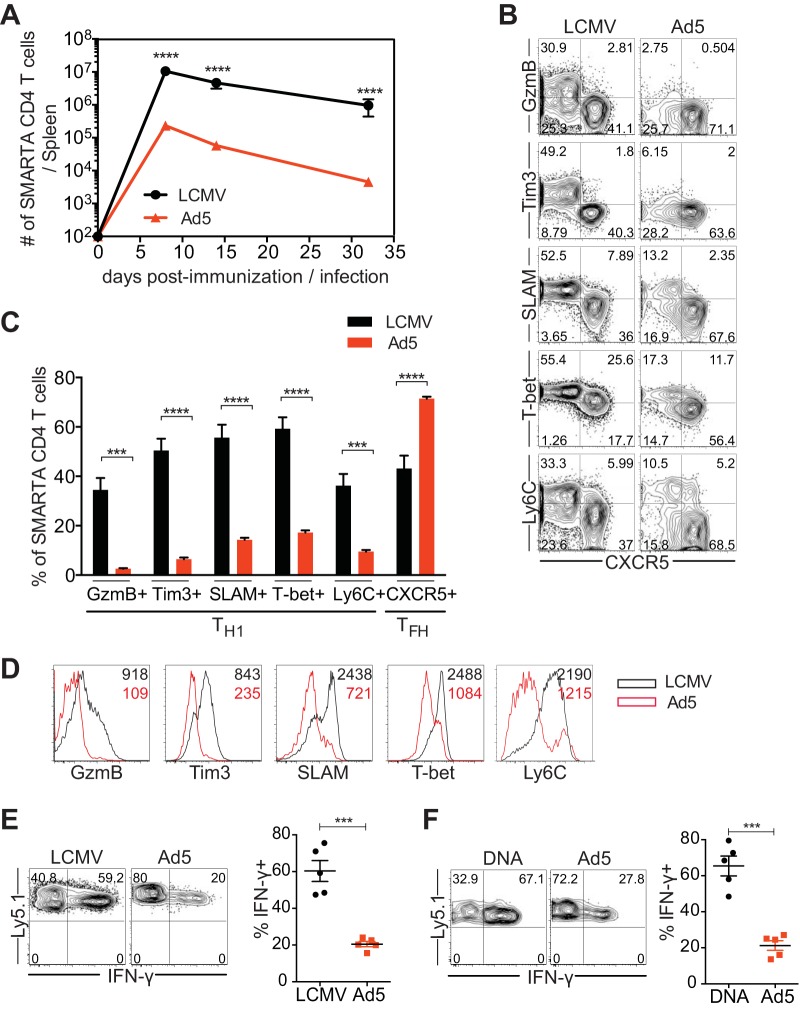
FIG 1.
Ad5 immunization leads to suboptimal Th1 differentiation. CD45.1+ SMARTA transgenic CD4 T cells specific for the LCMV GP66-77 epitope were transferred into C57BL/6 mice (CD45.2+) that were subsequently immunized with Ad5 vectors expressing full-length LCMV GP or infected with LCMV Armstrong strain. Congenically marked (CD45.1+) donor cells were analyzed in the spleen. (A) Kinetics of SMARTA CD4 T cells. (B to F) Analysis was performed at day 8 postimmunization or postinfection. (B) Representative fluorescence-activated cell sorting (FACS) plots, showing the phenotype of SMARTA CD4 T cells. (C) The frequency of SMARTA CD4 T cells expressing Th1 markers (granzyme B [GzmB], Tim3, SLAM, T-bet, Ly6C) or a Tfh marker (CXCR5). (D) Representative histograms of the indicated molecules expressed by SMARTA CD4 T cells. The numbers indicate the MFI of the indicated molecules. (E) Cytokine production of SMARTA CD4 T cells after ex vivo stimulation with GP61-80 peptide. (Left) Representative FACS plots show IFN-γ production of SMARTA CD4 T cells. (Right) The frequency of IFN-γ+ cells in SMARTA CD4 T cells. (F) SMARTA chimeric mice were generated and immunized intramuscularly with Ad5 or DNA vectors expressing full-length LCMV GP. Analysis was performed at day 8 postimmunization. Cytokine production was assessed after ex vivo stimulation with GP61-80 peptide. (Left) Representative FACS plots show IFN-γ production of SMARTA CD4 T cells. (Right) Frequency of IFN-γ+ cells in SMARTA CD4 T cells. Data are representative of 2 independent experiments with 4 to 5 mice per group per experiment. Error bars indicate standard errors of means. ***P < 0.001; ****P < 0.0001.