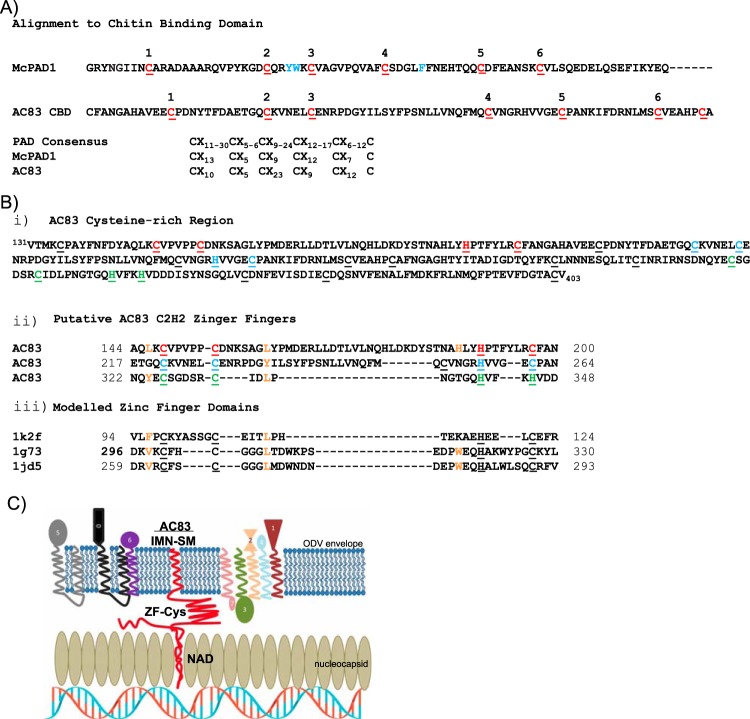
FIG 2.
Analysis of the protein domains within the AC83 cysteine-rich region. (A) Alignment of the AC83 region predicted by Zhu et al. (13) to contain a type II peritrophin A chitin binding domain with a known representative PAD chitin binding domain from Mamestra configurata PAD1 (McPAD1; GenBank accession number GU596430) (51). Cysteine residues comprising the PAD domain six-cysteine register are shown in red. Essential hydrophobic residues involved in chitin binding are shown in blue. Comparison of the McPAD1 and AC83 cysteine registers to that of the expanded consensus for PAD domains according to Tetreau et al. (52) is shown below. (B, i) Amino acid sequence of the AC83 cysteine-rich region (amino acids 131 to 403) showing cysteine residues (underlined) and cysteine and histidine residues that may form ZF domains (red, blue, and green). (ii) Alignment of AC83 regions that may form C2H2-type ZF domains with (iii) representative ZF domains from proteins with known structures. Protein Data Bank codes are provided at the left. Zinc ligands are underlined. Additional residues conforming to the consensus of Krishna et al. (23) are shown in orange. (C) Proposed working model of AC83 function in ODV and interaction with PIF proteins. The model shows the schematic cross-section of an ODV nucleocapsid adjacent to the ODV envelope. The AC83 domains are shown associated with the nucleocapsid (NAD) and with the envelope (INM-SM), and the predicted zinc finger Cys-rich domain (ZF-Cys) is shown interacting with the core PIF complex (PIF1-4) and the associated PIF proteins (PIF0, PIF6, and PIF7) and PIF5.