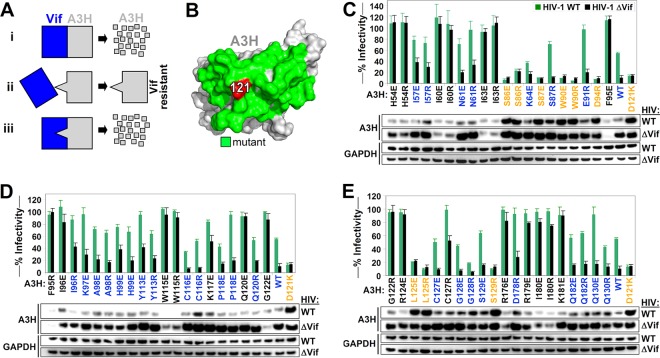
FIG 1.
Identification of the Vif binding site of A3H. (A) Overview of the approach used to map the Vif-A3H interaction. (Scenario i) Vif binds A3H, resulting in its degradation. (Scenario ii) An A3H with a mutation in the Vif-binding site is resistant to Vif and is not degraded. (Scenario iii) A Vif variant with a specific mutation to accommodate the A3H mutation would regain its ability to bind and degrade A3H. The mutation that makes A3H Vif resistant and the accommodating Vif mutation are likely directly interacting. (B) A3H residue 121 is indicated in red, and neighboring surface-exposed residues that were individually mutated for functional studies are colored in green. (C, D, and E) Single-cycle infectivity assay with 20 ng A3H R/E mutants, A3H WT, and A3H-D121K were cotransfected with 500 ng WT HIV or HIV ΔVif, the infectivity was analyzed in TZM-bl reporter cells, and A3H expression was assessed by Western blotting. A3H mutants that were resistant to Vif are colored in orange (HIV-WT/HIV-ΔVif infectivity ratio of <1.5 and restriction of HIV-ΔVif of >50%). Active A3H mutants that are sensitive to Vif are colored in blue (HIV-WT/HIV-ΔVif infectivity ratio of >1.5 and restriction of HIV-ΔVif of >50%). Inactive A3H mutants are indicated in black (restriction of HIV-ΔVif of <50%). The dashed line represents the 50% restriction cutoff. The infectivity of WT HIV and HIV-ΔVif in the absence of A3H was set at 100%, and the average relative infectivity values for a triplicate transfection are shown. Error bars represent the standard deviations.