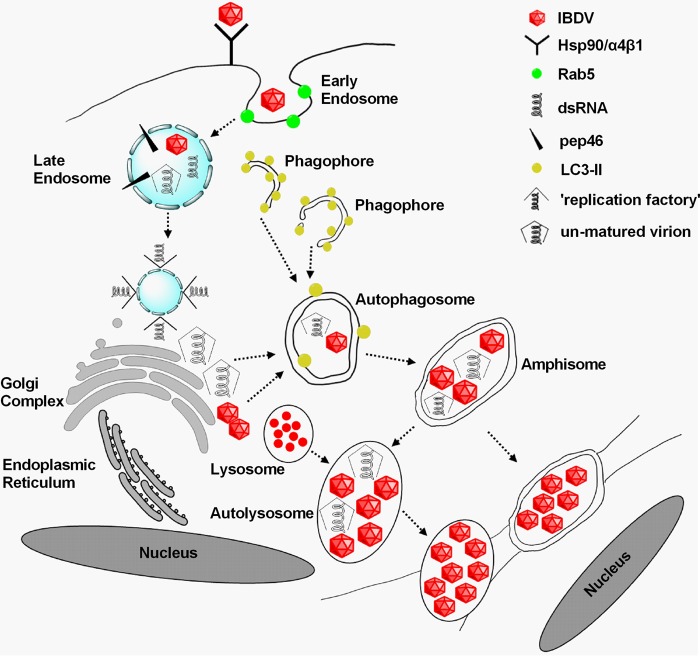
FIG 9.
Proposed model for IBDV replication cycle. Hsp90 and α4β1 integrin are components of the cellular receptor complex mediating IBDV infection (6, 7). Additionally, IBDV uptake involves macropinocytosis and trafficking to early endosomes in a Rab5-dependent manner, and a functional endocytic pathway is critical for viral infection (9). Uncoating of the virus occurs within the endosome in response to a low-pH environment (3). One viral peptide, pep46, generated during the processing of pVP2 maturation is suggested to deform biological membranes, leading to the formation of pores. The pores promote an exchange of small molecules between endosomal ghosts and the cytoplasm, allowing the initial transcription of the genome (3, 8). In addition, virus builds up its replication factory associated with the endosomal membrane (8). We revealed that IBDV induces typical double-membrane vesicles termed autophagosomes, which can fuse with vesicles of the endocytic pathway to form amphisomes and/or autolysosomes. Viral maturation is enhanced by the low-pH environment of amphisomes and/or autolysosomes. Virions are packaged within the cellular membrane, which facilitates fusion with the membranes of adjacent cells and delivers a large number of virions directly and rapidly into uninfected cells in cell-to-cell way. We propose that this may be a new mechanism for IBDV maturation, release, and reinternalization.