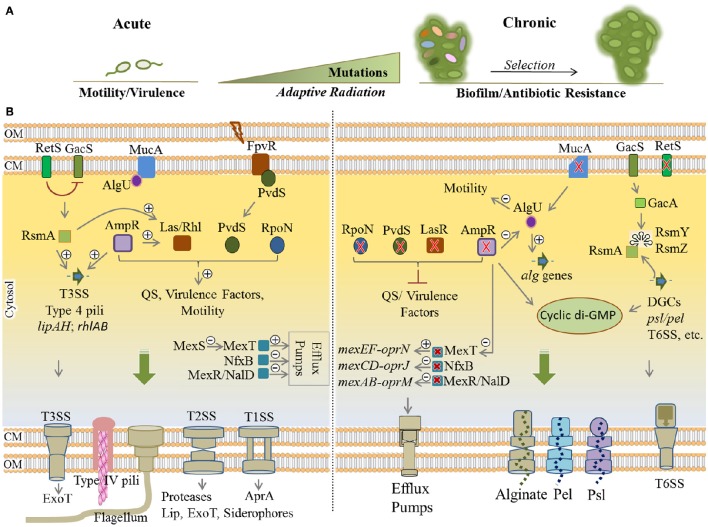
Figure 4.
Remodeling of regulatory networks in P. aeruginosa during adaptive radiation and transition from acute to chronic infections. (A) During pathogenesis, adaptation to the CF lung environment occurs through “adaptive radiation” where intense genetic mutations lead to diverse genotypes and phenotypes (colorful ellipsoids) within bacterial populations followed by the selection of colonizers. Mutational adaptation and selection of generations drive bacterial transition from acute to chronic traits. (B) Remodeling of key regulatory networks between acute and chronic infections occurs mainly via mutational adaptation in cognate genes. Mutated lasR, ampR, and retS genes are key determinants in this process by which QS, virulence factor production and motility are downregulated, while synthesis of cyclic di-GMP, exopolysaccharides and various multidrug efflux pumps are upregulated. Mutation of mucA results in a defect in MucA (anti-sigma factor) releasing AlgU (positive regulator of alginate operon) that induces overproduction of alginate and the mucoid phenotype. Of important acute traits are flagellum, type 4 pili, T1SS, T2SS, T3SS (types 1 to 3 secretion systems), ExoT (exotoxins), Lip (lipases), AprA (alkaline proteases). The type 6 secretion system (biofilm-associated and toxin-delivering device to other bacteria) and efflux pumps and the production of EPS are part of chronic traits which confer antibiotic resistance and/or mediate biofilm formation. Plus and minus signs represent positive and negative effect of transcriptional regulators, respectively. Red cross indicates mutagenesis. CM, cytoplasmic membrane; OM, outer membrane.