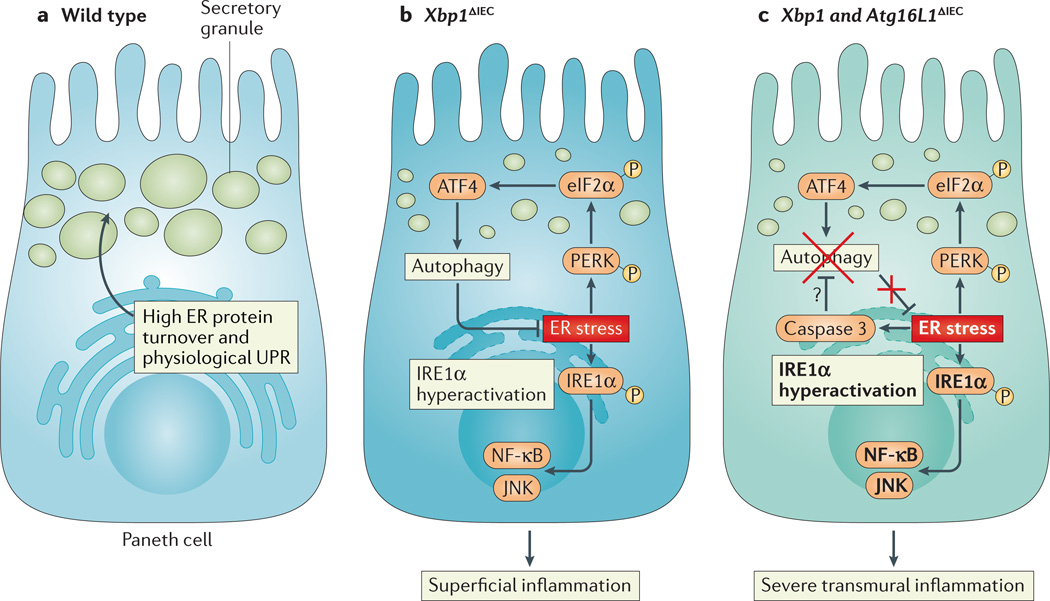
Figure 4. UPR signalling in intestinal epithelial cells.
a | Intestinal epithelial cells, particularly Paneth cells, have a well-developed endoplasmic reticulum (ER) to manage their high secretory demands, including the production of antimicrobial proteins such as lysozyme and defensins. b | Deletion of X-box binding protein 1 (XBP1) in the small intestinal epithelium (Xbp1ΔIEC mice) induces ER stress, resulting in hypomorphic Paneth cells that have signs of inositol-requiring enzyme 1α (IRE1α) hyperactivation and downstream activation of nuclear factor-κB (NF-κB) and JUN N-terminal kinase (JNK). The induction of autophagy through the PKR-like ER kinase (PERK)-eukaryotic translation initiation factor 2α (eIF2α)-activating transcription factor 4 (ATF4) axis of the unfolded protein response (UPR) alleviates ER stress. c | Simultaneous deletion of XBP1 and autophagy-related 16 like 1 (ATG16L1) in the small intestinal epithelium thus further increases ER stress and ER stress-induced inflammation. Hypothetically, ER stress, which induces caspase 3, could be an important pathway responsible for the degradation of ATG16L1 in patients carrying the Crohn’s disease risk allele ATG16L1T300A, which is prone to caspase 3-mediated cleavage.