Figure 6. NRG1/ErbB4 downstream signaling requires PKC dependent activation and membrane fusion–dependent exocytosis of AMPA receptors to restore excitatory inputs to deprived PV neurons.
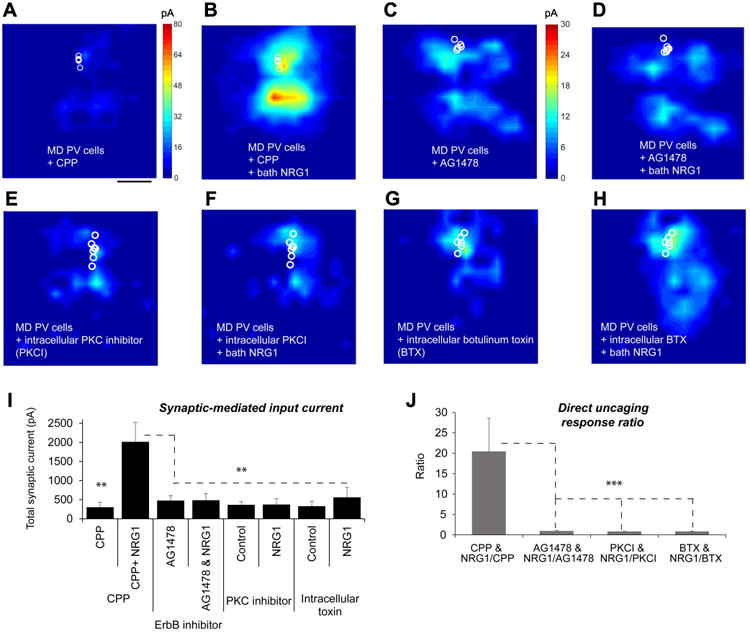
A-B, The presence of a NMDAR antagonist, CPP (3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid) does not affect NRG1 potentiation of excitatory inputs of 1-2 day deprived PV neurons. Group-averaged, excitatory input maps of L2/3 PV cells (n = 5 cells) in the CPP presence are shown for before (a) and during bath NRG1 (B). The spatial scale beneath (A) indicates 200 μm. C-D, An ErbB receptor tyrosine kinase inhibitor, AG1478 blocks NRG1 effects on 1-2 day deprived PV cells. Group-averaged, excitatory input maps of L2/3 PV cells (n = 10 cells) in the presence of AG1478 are shown for before (C) and during bath NRG1 (D). E-F, Intracellular application of a pseudosubstrate peptide inhibitor of protein kinase C (PKC (19-36), PKCI) prevents NRG1 induced effects on the recorded deprived PV cells. Group-averaged, excitatory input maps of L2/3 PV cells (n = 8 cells) in the intracellular presence of PKC (19-36) are shown for before (E) and during bath NRG1 (F). G-H, Intracellular application of botulinum toxin light chains (BTX) largely prevents NRG1 induced effects on the recorded deprived PV cells. Group-averaged, excitatory input maps of L2/3 PV cells (n = 7 cells) in the intracellular presence of BTX are shown for before (G) and during bath NRG1 (H). I, Summary data of average total synaptic input strength measured for L2/3 PV neurons under the specified conditions. ** indicate the significance levels of p < 0.01 (Mann–Whitney U tests). J, Direct uncaging response ratios of before and during bath NRG1 measured for L2/3 deprived PV neurons under the specified conditions. ***, p < 0.001 (Mann–Whitney U test).
