Abstract
The architectural diversity of nests in the passerine birds (order Passeriformes) is thought to have played an important role in the adaptive radiation of this group, which now comprises more than half of avian species and occupies nearly all terrestrial ecosystems. Here, we present an extensive survey and ancestral state reconstruction of nest design across the passerines, focusing on early Australian lineages and including members of nearly all passerine families worldwide. Most passerines build open cup-shaped nests, whereas a minority build more elaborate domed structures with roofs. We provide strong evidence that, despite their relative rarity today, domed nests were constructed by the common ancestor of all modern passerines. Open cup nests evolved from enclosed domes at least four times independently during early passerine evolution, at least three of which occurred on the Australian continent, yielding several primarily cup-nesting clades that are now widespread and numerically dominant among passerines. Our results show that the ubiquitous and relatively simple cup-shaped nests of many birds today evolved multiple times convergently, suggesting adaptive benefits over earlier roofed designs.
Keywords: Australia, evolution, nest, Passeriformes, phylogeny
1. Introduction
Birds exhibit an astonishing variety of nest designs, and in no group is this variety more striking than in the passerines. This group represents the largest evolutionary radiation of birds [1,2], comprising roughly 60% of extant avian species and encompassing a tremendous ecological and behavioural diversity. This life-history diversity is reflected in the architectural diversity of passerine nests, which range from simple grass mats to elaborate baskets and enclosed structures that can take weeks to construct [3,4]. Nest-building behaviour is considered one of the major innovations allowing passerines to diversify into such a wide variety of ecological niches and terrestrial habitats [5–7].
All passerine bird species construct or adopt nests of some description in which to lay eggs and care for nestlings, and different designs have different advantages and disadvantages [8,9]. Open cup-shaped nests are the most common design [5], having the advantage of relatively simple construction but the disadvantage of potentially exposing eggs, nestlings, and parents to predators and climatic elements [10,11]. A smaller proportion of species build domed nests with roofs, which can take longer to construct but provide greater concealment and insulation [3,5]. Both of these nest types may be placed on the ground, in vegetation, or in some cases attached to vertical surfaces. Birds may also nest in tree hollows or underground burrows, either by adopting pre-existing cavities or by excavating their own [3,4]. Many cavity nesters line the bottoms of their cavities with grass or similar material, thus forming a cup, whereas others are known to construct domed nests within cavities [12]. Cavities provide enhanced protection for young, as in free-standing domed nests, but they are also limited in availability and energetically expensive to create [5].
Nests can vary among and even within species in some aspects, such as size and the particular building materials used [11,13]. Yet, the general design of a nest, for instance whether it is constructed as a cup or a dome and whether or not it is built within a cavity, is often relatively invariant within and across closely related species and therefore conserved over evolutionary timescales. Indeed entire passerine families are known to exhibit the same basic nest type [12], and nest structural characters have long been used in taxonomic classification [4]. Nest evolution has been investigated in phylogenetic studies of several passerine families with particularly diverse nests, including the swallows and martins (Hirundinidae [14]), ovenbirds (Furnariidae [15,16]), babblers (Timaliidae [17]), and New World blackbirds (Icteridae [18]). However, the evolution of nest-building behaviour at broader phylogenetic and geographical scales has not been quantitatively assessed.
Relative to morphological traits, we know little about how complicated, largely unlearned behavioural sequences evolve [19], including the behaviours involved in avian nest building [5]. Differences in nest architecture among species are thought to reflect differences in the precisely coordinated motor patterns and underlying genetic programmes of nest builders, so studying nest evolution provides a means for understanding the evolution of complex behaviour in general [20]. The domed nests of many passerine species are hypothesized to have evolved from simpler open cups, based partly on the prevalence of cup nests today but also on the assumption that behavioural sequences tend to evolve cumulatively [3,5].
Here, we test that hypothesis by reconstructing the evolution of nest architecture across the Passeriformes, including nearly all recognized passerine families, with the central goal of reconstructing ancestral nest features in the earliest passerine lineages. We reconstructed nest shape (cup or dome), nest location (in cavities, on vegetation, on the ground, or attached to vertical surfaces), and the likely geographical areas in which ancestral changes occurred. Previous molecular phylogeographic studies have indicated Australasia as the origin of most extant passerine lineages [1,2,21–23]. The oscine passerines (songbirds), which alone comprise nearly half of all bird species, arose on the Australian continent [2], following earlier Gondwanan divergences of the suboscine passerines and New Zealand wrens (Acanthisittidae) [1,21]. Our study of nest evolution therefore included two stages: (i) a detailed survey and ancestral state reconstruction of nest design among Australian passerine species and (ii) a broader family-level analysis of nest evolution across 124 passerine families worldwide (taxonomy following the IOC 6.1 World Bird List [24]). Both analyses yielded complementary and largely identical results, providing a framework for future investigations into the factors influencing historical changes in nest structure.
2. Methods
(a). Scoring nest characteristics
We gathered information on Australian passerine nests from published sources [12,25–28] and by visiting the Australian National Wildlife Collection (Canberra, Australia: http://www.csiro.au). Our survey included 315 extant species from 39 families known to breed in Australia and outlying islands [29], excluding introduced taxa and one poorly known native species Glycichaera fallax (electronic supplementary material, table S1). Although our focus on the Australian continent omitted some phylogenetically important taxa in other regions of Australasia (e.g. New Zealand, Papua New Guinea), Australia includes representatives of most Australasian families and provides the most inclusive and detailed sampling of passerine nest characteristics [28].
For our broader family-level analysis, whenever possible we relied on the family-wide descriptions of nests and geographical ranges provided in Handbook of Birds of the World Alive (HBW) [12]. Our analysis included 124 of the 128 extant families in the Passeriformes as recognized by the IOC 6.1 [24] (electronic supplementary material, table S2), excluding just four poorly known monotypic families (Elachuridae, Hylocitreidae, Pityriaseidae, Urocynchramidae). We scored a family as having a particular nest characteristic if fewer than 5% of species were known to exhibit any alternative character state (91 families total), a threshold intended to account for undescribed species and potential errors in reporting. Thus, for example, members of the honeyeaters (Meliphagidae) were scored as having open cup-shaped nests despite the fact that two of the 186 known species (1.1%) are reported to build pendulous enclosed domes [12]. If two or more nest character states were each reported in 5% or more of family members, we assigned the family score as ‘variable’ (33 families total). Of these 33 variable families, all but one (Melampittidae) are members of either the New World suboscines or the Passerida. Whenever necessary we surveyed individual species within families to determine the distribution of nest characteristics (79 families overall), especially when taxonomies in HBW differed from those of IOC 6.1.
We scored nest characteristics according to nest shape (cup or dome) and location (in cavities, on vegetation, on the ground, or attached to vertical surfaces). We further combined these scores to assess nests as either open or enclosed (by a constructed roof, a cavity or both) to distinguish open cup nests from others. Taxa that do not build their own nests, such as obligate brood parasites (e.g. indigobirds and whydas, Viduidae), were scored as unknown.
We categorized nest shapes as either cups or domes following Collias [5]. Each of these two types includes a variety of elaborated and specialized forms [3,4], but nonetheless they are easily distinguished. Cup nests are any in which the upper portion is exposed, including simple concave platforms, hemispherical bowls, and suspended baskets, which require a range of different skills to construct [3,4]. Domed nests are any in which the upper portion is enclosed by a constructed roof, including globular structures and pendulous nests with side entrances. Some domed nests have long entrance tunnels [12].
Cavity nesters included taxa that lay their eggs in tree holes, rock crevices, or underground burrows. We recognized obligate cavity nesters as opposed to those that nest in cavities only occasionally and opportunistically (e.g. in nest-boxes); however, we did not distinguish taxa that adopt pre-existing cavities from those that excavate their own, in part because this is unknown in some cases and in part because some taxa appear to do both [12]. We distinguished species that nest directly on the ground from those that build their nests on any type of vegetation, including grasses, shrubs, and trees.
For the family-level analysis, we also recorded geographical ecozone (Australasian, Afrotropic, Indomalayan, Palaearctic, Nearctic, and Neotropic) as delineated by Udvardy [30] and based on ranges in HBW [12]. Families often span multiple ecozones, so for our evolutionary reconstructions we consolidated these regions into just four geographical character states: Australasian, Old World (Afrotropic, Indomalayan, Palaearctic), New World (Nearctic, Neotropic), and worldwide.
(b). Phylogenetic analyses
To reconstruct the evolution of nest structure, we overlaid our character scores onto three exhaustive passerine phylogenies, each of which included all available genetic sequence data at the time of publication [31–33]. We used a majority-rule consensus of 1 000 randomly sampled phylogenies from Jetz et al. [31,34], using the Hackett backbone and including only taxa with sequence data [35]. We also mapped our data onto the passerine trees by Hugall & Stuart-Fox [32] and Burleigh et al. [33]. The three trees differed slightly in topology, allowing us to ensure that our results were not based on any one phylogenetic hypothesis.
For our Australian analysis, the trees from Jetz et al. [31], Hugall & Stuart-Fox [32], and Burleigh et al. [33] included 260, 281, and 274 Australian species, respectively, of the 315 included in our study (24 species were not included in any of the trees). For our passerine-wide family-level analysis, we selected one representative species from each of 124 passerine families to be included in our phylogenies (electronic supplementary material, table S2). We chose representative species that were included in all three molecular trees [31–33] with a preference for species with more sequence data.
We used three ancestral state reconstruction models to determine likely ancestral nest characteristics: unordered parsimony, Markov k-state one-parameter (Mk1) maximum-likelihood, and asymmetrical Markov k-state two-parameter (AssymMk2) maximum-likelihood. We reconstructed ancestral states and compared the overall agreement of these models using Mesquite v. 3.04 [36], which allowed us to test the robustness of our results to different evolutionary assumptions. Whereas parsimony is the simplest model, resolving ancestral states that minimize the number of character changes on a tree, maximum-likelihood models estimate the uncertainty associated with character reconstruction and take into account branch lengths, which represent levels of species divergence. Both parsimony and Mk1 maximum-likelihood assume that changes among character states are equally probable, whereas AssymMk2 maximum-likelihood allows probabilities of gains and losses to differ [36]. This latter model can only be applied to binary characters, those with no more than two states, so it was not used in reconstructing nest location or geographical region.
We investigated whether differences in net diversification rates (the difference between speciation and extinction rates) and transition rates between nest types could affect historical reconstructions using the binary-state speciation and extinction (BiSSE) model [37], as implemented in the Diverse package in Mesquite [36]. Relatively low rates of diversification in dome-nesting taxa, for example, could support a cup-nesting ancestor if transitions from cup nests to domed nests occurred sufficiently frequently (e.g. [38]).
Maximum-likelihood and BiSSE models do not allow uncertainty in character scores. Thus, for taxa that exhibited multiple potential states and were scored as ‘variable’, either within Australian species or among species within passerine families, we performed separate analyses each favouring a particular character state (e.g. favouring cups or favouring domes). This, in addition to our use of multiple phylogenies and reconstruction models, generated a range of rate estimates and probability values for each ancestral state.
3. Results
Approximately a third of Australian passerine species (106/315 or 33.7%) build domed rather than cup-shaped nests (table 1), and likewise 11 of the 30 passerine families endemic to Australasia (36.7%) build primarily domed nests (electronic supplementary material, table S2), which is a slightly higher proportion of dome-builders than occurring elsewhere in the world (χ2 = 4.1, p = 0.043). Worldwide only 35 of 123 nest-building families (28.5%) include members that construct domed nests, either exclusively (23 families) or predominantly (12 families) (table 1). Most passerine families (71.5%) build cup-shaped nests.
Table 1.
Numbers (and proportions) of Australian passerine species and world passerine families with various nest characteristics.
| total | shape |
location |
exposure |
||||||
|---|---|---|---|---|---|---|---|---|---|
| cup | dome | cavity | vegetation | ground | cliff | open | enclosed | ||
| Australian species | 315 | 209 (0.66) | 106 (0.34) | 18 (0.06) | 268 (0.84) | 26 (0.08) | 3 (0.01) | 195 (0.62) | 120 (0.38) |
| world families | 123a | 88 (0.72) | 35 (0.28) | 16 (0.13) | 100 (0.81) | 6 (0.05) | 1 (0.01) | 77 (0.63) | 46 (0.37) |
| more than 95% of members | 76 (0.62) | 23 (0.19) | 10 (0.08) | 87 (0.71) | 3 (0.02) | 1 (0.01) | 66 (0.54) | 36 (0.29) | |
| 50–95% of members | 12 (0.10) | 12 (0.10) | 6 (0.05) | 13 (0.11) | 3 (0.02) | 0 (0.00) | 11 (0.09) | 10 (0.08) | |
aViduidae are brood parasitic and do not build nests, so they are not included in the total number of world families.
Although they are relatively uncommon today, domed nests were found to be the most likely ancestral passerine nest design in all of our reconstructions of ancestral character states, regardless of reconstruction method and regardless of whether we included just Australian species or passerine families worldwide (table 2). Among Australian taxa, parsimony analyses on all three phylogenies unequivocally reconstructed a domed nest as the ancestral state, as did both maximum-likelihood models (Mk1 and AssymMk2) with probabilities ranging from 95.3 to 98.8% and with strong support using a likelihood decision threshold of 2.0 (figure 1 and table 2). Likewise, our broader analysis including oscine and suboscine passerine families worldwide invariably reconstructed domed nests as the most likely ancestral state, regardless of how we scored variable character states and regardless of whether we used parsimony, Mk1 maximum-likelihood (68.7–80.7%), or AssymMk2 maximum-likelihood (90.6–96.6%) (table 2).
Table 2.
Probabilities of ancestral states from multiple phylogenetic reconstructions of nest characteristics. All ancestral state reconstructions were performed on three phylogenies [31–33] and using three different reconstruction models (unordered parsimony, Mk1 maximum-likelihood, and AssymMk2 maximum-likelihood), which in turn were run multiple times with variable character states favouring each possible state, resulting in a range of probability values.
| model | shape |
location |
exposure |
||||||
|---|---|---|---|---|---|---|---|---|---|
| cup | dome | cavity | vegetation | ground | cliff | open | enclosed | ||
| Australian species | parsimony | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 |
| Mk1 | 0.03–0.05 | 0.95–0.97a | 0.0–0.01 | 0.97–0.99a | 0.0–0.01 | 0.0–0.01 | 0.0–0.01 | 0.99–1.00a | |
| AssymMk2 | 0.01–0.02 | 0.98–0.99a | — | — | — | — | 0.0–0.01 | 0.99–1.00a | |
| world families | parsimony | 0.00 | 1.00 | 0.50 | 0.50 | 0.00 | 0.00 | 0.00 | 1.00 |
| Mk1 | 0.19–0.31 | 0.69–0.81 | 0.04–0.09 | 0.88–0.95a | 0.01–0.02 | 0.01–0.02 | 0.08–0.27 | 0.73–0.92a | |
| AssymMk2 | 0.03–0.09 | 0.91–0.97a | — | — | — | — | 0.01–0.26 | 0.74–0.99a | |
aAncestral states strongly supported by a likelihood decision threshold of 2.0.
Figure 1.
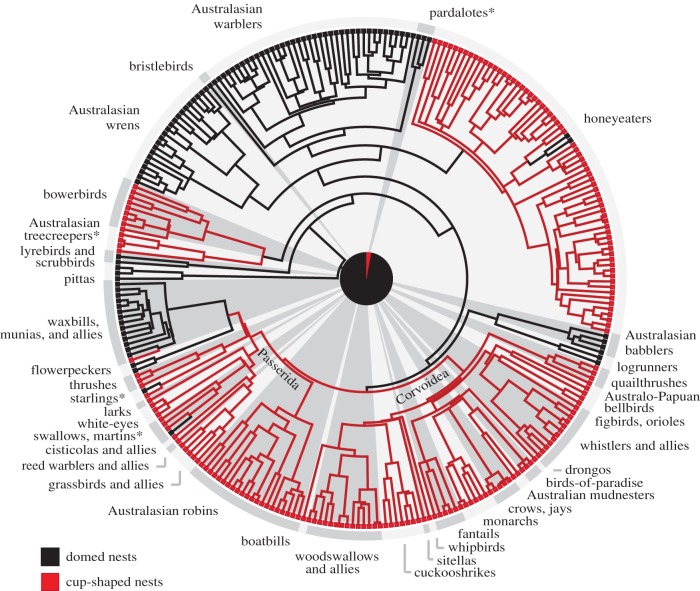
Ancestral state reconstruction of nest shape on a DNA-based phylogenetic tree of 281 Australian passerine species [32]. Although cup-shaped nests (red) are more common than domed nests (black) among species today (187 or 66.5% of species on the tree build cup nests), multiple analyses reconstructed domed nests as the ancestral state. The pie chart in the centre shows the probability of a domed nest in the common ancestor (97% likelihood on this phylogeny using Mk1 maximum-likelihood, strongly supported by a likelihood decision threshold of 2.0). Reconstructions using different evolutionary models and alternative trees [31,33] gave nearly identical results (also see electronic supplementary material, figure S1). Asterisks indicate families that are primarily cavity nesters.
Net diversification rates did not differ consistently between cup-nesting and dome-nesting Australian species, and these values varied widely depending upon how variable character states were scored in passerine families (electronic supplementary material, table S3; also see Rabosky & Goldberg [39] for problems in such analyses). However, transition rates from domed nests to cup nests were consistently higher than the reverse, providing strong additional evidence that the ancestral passerine nest was domed.
Maximum-likelihood reconstructions indicated that the ancestral passerine nest was built on vegetation such as trees or shrubs (97.5–99.4% likelihood using Australian species and 87.7–94.7% likelihood using world families) rather than in cavities or on the ground (table 2). Most extant passerines (more than 81%) likewise build their nests on vegetation today (table 1). Only 16 passerine families (13% of the total) tend to nest in cavities, 12 of which build cup-shaped nests and four of which build domed nests within cavities. Australian taxa reflect this broader pattern, with a minority of species (6%) nesting in cavities, some of which build cups (12 species) and some of which build domes (six species). Nevertheless, despite strong maximum-likelihood support for a vegetation-nesting ancestor, parsimony reconstructions were equivocal about whether the ancestral passerine nest was placed within cavities or on vegetation (table 2). The sister group of all other passerines, the New Zealand wrens (Acanthisittidae), build domed nests within cavities [25], and cavity nesting is also common in the sister group of the Passeriformes, the parrots (Psittaciformes) [12,31–33], so it seems likely that cavity nesting occurred in ancestors of the Passeriformes. Yet cavity nesting was almost certainly absent in the ancestral lineage following the split with Acanthisittidae and leading to the rest of the passerines (less than 1% likelihood in all of our analyses).
Ground nesting, like cavity nesting, has evolved among scattered taxa throughout the passerine phylogeny, predominating in only six passerine families (4.8% of the total) (table 1). Ground nesters are more likely to build cups than domes: four of six ground-nesting families and 14 of 26 Australian ground-nesting species tend to build open cups rather than domed nests, not supporting previous suggestions that roofed nests occur especially frequently in ground nesters [3,5,17].
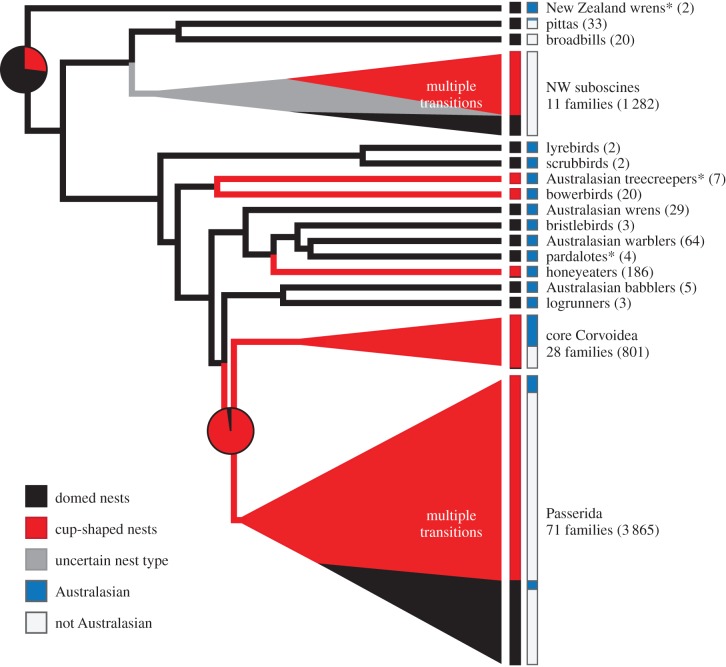
Open (non-cavity) cup-shaped nests evolved from enclosed dome nests at least four times independently during the early history of the passerines (figure 2): in the New World suboscines, the bowerbirds (Ptilonorhynchidae), the honeyeaters (Meliphagidae), and a worldwide radiation including the majority (approx. 74%) of extant passerine species. This worldwide radiation in turn includes two diverse and cosmopolitan clades: the core Corvoidea (crows and allies) and the much larger Passerida (warblers, finches, and a variety of other songbirds) [1,2,40]. Our parsimony and maximum-likelihood analyses invariably reconstructed the common ancestor of these two clades as having a nest that was cup-shaped (all with more than 96% likelihood) and built on vegetation (more than 99% likelihood), together indicating that open cup nests appeared prior to the explosive evolutionary diversification of these taxa, which included multiple reappearances of domed nests in the Passerida (figure 2).
Figure 2.
Schematic showing major evolutionary transitions to cup-shaped nests (red) from domed nests (black) among passerine families worldwide (grey indicates ambiguous reconstructions). Major evolutionary radiations of the New World (NW) suboscines, core Corvoidea, and Passerida are condensed and shown as triangles (all with more than 93% RAxML bootstrap support [32]). Boxes and bars on the right show approximate proportions of species with cup-shaped nests (red) and domed nests (black) in each group, as well as the proportions of taxa located within (blue) or outside (white) Australasia. Multiple evolutionary transitions between cup-shaped and domed nests have occurred in the NW suboscines and the Passerida (see electronic supplementary material, figure S2), so continuity of red or black triangles and bars in these groups should not imply monophyly of cup- or dome-nesting taxa. Numbers of species in each group are given in parentheses (based on IOC 6.1 [24]), and asterisks indicate families that are primarily cavity nesters. Pie charts show lowest Mk1 maximum-likelihood values for cup-shaped and domed nests at two nodes on this tree [32], with a minimum 73% likelihood that the ancestor of all passerines had an enclosed dome nest and a minimum 96% likelihood that the Corvoidea-Passerida ancestor had a cup-shaped nest.
Our reconstructions were more ambiguous about the origins of cup-shaped nests in the New World suboscines (figure 2). All parsimony analyses supported a cup-nesting ancestor for this group, whereas Mk1 and AssymMk2 maximum-likelihood values varied dramatically depending upon how we scored variable character states, from 94.9% likelihood of a cup to 99.4% likelihood of a dome. Cup nests either evolved once in the ancestor of the group followed by multiple reversals to domed nests, or they evolved later at least four times, or some combination of these scenarios. Regardless, all of our reconstructions showed that the open cup nests of extant New World suboscine taxa evolved from dome-nesting ancestors.
Including the geographical ranges of families (Australasian, Old World, New World, or worldwide) as character states in our reconstructions indicated that the dome-nesting ancestor of all modern passerines was Australasian (electronic supplementary material, figure S2). This ancestral state was supported in all of our reconstructions by parsimony and by high maximum-likelihood values (82.2–95.5%) using a likelihood decision threshold of 2.0. Our reconstructions further indicated that, outside of the New World suboscines, all three initial transitions from domed to open cup nests occurred on the Australian continent, evident in our analysis of Australian species (figure 1) and in our broader analysis of passerine families across the world (figure 2). The bowerbirds and the honeyeaters, for instance, evolved open cup-shaped nests independently, and both families are Australasian endemics with origins in Australia [22,41,42]. The cup-nesting common ancestor of the Corvoidea-Passerida clade was also placed in Australasia in our reconstructions, with parsimony and maximum-likelihood values of more than 99% at this node, again in line with previous studies showing an Australian origin [1,2,21,22]. We were unable to determine the geographical origin of cup nests in New World suboscines, a group with ancient Gondwanan relationships to other passerines and which today is almost entirely confined to the Neotropics [1,12,21].
Multiple evolutionary reversals to enclosed nests, with roofs, or in cavities, or both, have occurred since the initial transitions to open cups (electronic supplementary material, figure S2). For example, within the honeyeaters pendulous domed nests evolved from cup-nesting ancestors in the genus Ramsayornis (figure 1), and within the core Corvoidea clade a domed nest has been reported only in the little-known species Melampitta lugubris [43]. More transitions between open and enclosed nests have occurred in the New World suboscines (figure 2), in which at least six of 11 families include species with domed or cavity nests. But it is in the Passerida, recognized as the largest avian radiation [2], that nest diversification appears most extensive. At least 40 of 71 families in this group include taxa with enclosed nests, and our reconstructions revealed at least 38 independent transitions among open cup, domed, and cavity nests among families. Additional evolutionary transitions have presumably occurred within families, including transitions from enclosed nests back to cups, given how many Passerida and New World suboscine families are known for their diverse nest designs [14–18] and were scored as having variable nest characteristics in our analysis. The Passerida and the New World suboscines together include over 80% of extant passerine species [24], and they account for most of the world's nest-building diversity today [3–6].
4. Discussion
Our findings illustrate how the current prevalence and relative complexity of a trait may not necessarily reflect the order of events in its evolutionary history. Open cup-shaped nests are common among passerine birds today and appear in much the same form across widely divergent taxa. They are generally relatively simple in comparison to roofed structures [5,10], and in fact many dome-nesting birds build their nests by initially constructing a cup [3,4]. Based on these observations alone it seems self-evident that domed nests evolved from simpler forms that were open above. Indeed, Collias [5] noted that ‘the open nest, by the criteria of commonality (general occurrence), relative simplicity, and ontogeny, appears closer to the ancestral type of bird nest than does the domed nest’.
Yet, based on ancestral state reconstructions using multiple methods and phylogenies and with variable character states scored multiple ways, we found that the ancestral passerine nest was domed. This and other early passerine lineages (excluding suboscines) were also found to be Australasian, as shown in a variety of previous phylogeographic analyses of early passerine diversification [1,2,21–23]. Many of the most phylogenetically distinct passerine lineages are endemic to Australasia, such as the New Zealand wrens, lyrebirds (Menuridae), and scrubbirds (Atrichornithidae), and most of these ancient Australasian endemics build domed nests presumably reflecting the ancestral form (figure 2). Passerines include a higher proportion of dome-nesting taxa than does any other avian group [3,5], and within passerines our study shows that a higher proportion of dome-nesting taxa occur in Australasia than in any other region of the world. Nevertheless, dome-nesters constitute only about a third of Australian passerine taxa today (table 1). Open-cup-nesting lineages appeared more recently on the passerine tree, yet they now comprise the majority of extant passerine species. Taken together, our results suggest that dome-nesting passerines were more common in the evolutionary past.
Open cup nests evolved from ancestral domed nests multiple times independently, probably just once each in the ancestors of the bowerbirds, honeyeaters, and Corvoidea-Passerida clade, and possibly more than once in the New World suboscines (figure 2). The open nests of these groups are strikingly similar in overall structure [3,4], yet our reconstructions reveal that they evolved convergently, both with each other and with non-passerine cup-building birds. Transitions from domed to cup nests have occurred more often than the reverse, and these repeated losses of roofs presumably reflect repeated losses of the behavioural sequences involved in building them. Likewise, similarities among the more recently and independently derived domed nests of some Passerida taxa (e.g. Estrildidae, Troglodytidae) and those of Australasian endemics (e.g. Maluridae) are also the result of convergent evolution, but through independent gains in roof-building behaviours rather than losses. This should serve as a reminder that, even in traits as complex and as evolutionarily conserved as passerine nest designs, overall similarities can be misleading about evolutionary relationships.
The evolution of open cup nests in bowerbirds is particularly interesting considering that males in this group court females by building elaborate bowers [42], with walls and/or roofs that can be strikingly reminiscent of the domed nests presumably constructed by ancestors. Female bowerbirds build open cup nests on their own [3,12]. Bower building and nest building have long been recognized as homologous behaviours (e.g. [3]). Our results suggest that, in at least some of their architectural features, the cup nests of females differ more from ancestral nests than do the bowers of males.
Divergence time estimates of several previous studies [2,22,31,41,44] suggest that open cup nests first appeared in Australia during the Eocene, following its separation from Antarctica when Australia was much less arid than it is today and wet forests were widespread [41]. The honeyeaters are thought to have diverged from other Australian passerines in the mid-Eocene [22,41], and Passerida taxa probably radiated from Australia during roughly the same time period between 40 and 47 million years ago [2,22,31,44]. The timing of the origin of cup-shaped nests in the bowerbirds is less clear because our reconstructions could not resolve whether this nest shape evolved before or after the divergence of bowerbirds and their sister group the Australasian treecreepers, which build similar woven cups, although placed within cavities [45]. Given the presumed timing of the bowerbird-treecreeper divergence in the Eocene [22], however, neither scenario of nest evolution precludes an appearance of open (non-cavity) cup nests in the bowerbirds that is somewhat contemporaneous with changes in the honeyeaters and the Corvoidea-Passerida clade. This roughly simultaneous evolution across three distinct Australian lineages would suggest a common cause.
What might explain these ancient transitions from enclosed domes to open cups? On the one hand, cup-shaped nests can be constructed more quickly and with fewer materials than roofed nests [8,10], so they may be more easily replaced following a loss [46]. Open nests might also allow parents to more easily escape from predators [5]. However, on the other hand, open nests provide less concealment and protection than do domes or cavities, leaving parents and offspring more exposed to predators and environmental changes [8,9]. Both open and enclosed nest designs present trade-offs in costs versus benefits, implying that these parameters have changed over evolutionary time. Selection for open nests may have occurred with the emergence of new nest predators or parasites or with changing climates and habitats, as occurred in Australia during the Eocene [41]. Open nests may have also coevolved with cryptic plumage in incubating females and losses of female song [47,48]. Detailed comparative studies of passerine families with both types of nest (e.g. [17,18]), or experiments with the few species known to build cups or domes in different parts of their ranges (e.g. Cisticola exilis [12]), might provide further insights into the potential selective mechanisms underlying historical changes between these two distinct nest forms.
Although we found no consistent differences in overall diversification rates based on nest shape, it is nevertheless interesting that each of the early transitions to open cup nests appears to have been followed by more descendent taxa in comparison to enclosed-nesting sister groups (figure 2). Bowerbirds are nearly three times as speciose as Australasian treecreepers, which nest in cavities; honeyeaters include more species than all of their Australasian (non-Corvoidea-Passerida) dome-nesting relatives combined; and the New World suboscines include nearly 25 times as many species as their closest relatives the broadbills and pittas, known as the Old World suboscines, which all build domed nests. In the Corvoidea-Passerida clade, open cup nests evolved immediately prior to the emergence and subsequent explosive radiation of this group worldwide [1,2]. Open cup-shaped nests therefore may have been a key innovation during early passerine evolution, providing adaptive benefits over earlier enclosed designs.
5. Conclusion
The complexity of avian nests, along with the skills required to build them, has long been viewed as both a challenge and an opportunity in evolutionary biology [8,9,13,49]. Our study reveals a surprising level of order underlying the seemingly bewildering diversity of passerine nests, with high levels of evolutionary conservatism in basic nest design and with some passerine families, particularly in Australasia, building nests that appear little changed over tens of millions of years. This pattern of evolutionary stability punctuated by relatively few innovative events resembles the evolution of other complex, largely innate behaviours in birds [42,50], perhaps pointing to the intricate and modular nature of the genetic influences underlying such behavioural sequences [20]. We hope that our phylogenetic framework for the evolution of passerine nest diversity provides a foundation for future investigations into the cognitive, genetic, and ecological determinants of one of the best known extended phenotypes in the natural world [51].
Supplementary Material
Supplementary Material
Acknowledgements
We thank Andrew Hugall for advice and for generously sharing his passerine phylogeny and Leo Joseph for providing access to the avian nest collection at the ANWC. Karan Odom provided invaluable advice on using Mesquite and constructing figures, and Daisy Duursma provided help with data management. Becky Cramer and two anonymous reviewers made valuable comments on the manuscript.
Data accessibility
Nest data for Australian passerine species (electronic supplementary material, table S1) and world passerine families (electronic supplementary material, table S2), results of BiSSE analyses (electronic supplementary material, table S3), and ancestral state reconstructions of nest characteristics (electronic supplementary material, figure S1 and electronic supplementary material, figure S2) have been uploaded as part of the electronic supplementary material.
Authors' contributions
J.J.P. and S.C.G. conceived the study and collected data on nest characteristics. J.J.P. performed and interpreted the analyses and wrote the manuscript with input from S.C.G. Both authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
Work in Australia by J.J.P. was supported by a Faculty Development Grant from St. Mary's College of Maryland. S.C.G. was supported by a Future Fellowship from the Australian Research Council (FT130101253).
References
- 1.Barker FK, Barrowclough GF, Groth JG. 2002. A phylogenetic hypothesis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proc. R. Soc. Lond. B 269, 295–308. ( 10.1098/rspb.2001.1883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker FK, Cibois A, Schikler PA, Feinstein J, Cracraft J. 2004. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. USA 101, 11 040–11 045. ( 10.1073/pnas.0401892101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collias NE, Collias EC. 1984. Nest building and bird behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Hansell MH. 2000. Bird nests and construction behaviour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Collias NE. 1997. On the origin and evolution of nest building by passerine birds. Condor 99, 253–270. ( 10.2307/1369932) [DOI] [Google Scholar]
- 6.Olson SL. 2001. Why so many kinds of passerine birds? Bioscience 51, 268–269. ( 10.1641/0006-3568(2001)051%5B0268:WSMKOP%5D2.0.CO;2) [DOI] [Google Scholar]
- 7.Bennett PM, Owens IPF. 2002. Evolutionary ecology of birds: life history, mating systems and extinction. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC. 2014. The design and function of birds’ nests. Ecol. Evol. 4, 3909–3928. ( 10.1002/ece3.1054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeming DC, Reynolds SJ (eds). 2015. Nests, eggs, and incubation. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Mainwaring MC, Hartley IR. 2013. The energetic costs of nest building in birds. Avian Biol. Res. 6, 12–17. ( 10.3184/175815512X13528994072997) [DOI] [Google Scholar]
- 11.Heenan CB. 2013. An overview of the factors influencing the morphology and thermal properties of avian nests. Avian Biol. Res. 6, 104–118. ( 10.3184/003685013X13614670646299) [DOI] [Google Scholar]
- 12.del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E. 2016. Handbook of the birds of the world alive. Barcelona, Spain: Lynx Edicions; See http://www.hbw.com/ (accessed March 2016). [Google Scholar]
- 13.Guillette LM, Healy SD. 2015. Nest building, the forgotten behaviour. Curr. Opin. Behav. Sci. 6, 90–96. ( 10.1016/j.cobeha.2015.10.009) [DOI] [Google Scholar]
- 14.Winkler DW, Sheldon FH. 1993. Evolution of nest construction in swallows (Hirundinidae): a molecular phylogenetic perspective. Proc. Natl Acad. Sci. USA 90, 5705–5707. ( 10.1073/pnas.90.12.5705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zyskowski K, Prum RO. 1999. Phylogenetic analysis of the nest architecture of Neotropical ovenbirds (Furnariidae). Auk 116, 891–911. ( 10.2307/4089670) [DOI] [Google Scholar]
- 16.Irestedt M, Fjeldsa J, Ericson PGP. 2006. Evolution of the ovenbird-woodcreeper assemblage (Aves: Furnariidae)—major shifts in nest architecture and adaptive radiation. J. Avian Biol. 37, 260–272. ( 10.1111/j.2006.0908-8857.03612.x) [DOI] [Google Scholar]
- 17.Hall ZJ, Street SE, Auty S, Healy SD. 2015. The coevolution of building nests on the ground and domed nests in Timaliidae. Auk 132, 584–593. ( 10.1642/AUK-15-23.1) [DOI] [Google Scholar]
- 18.Drury JP, Burroughs N. 2016. Nest shape explains variation in sexual dichromatism in New World blackbirds. J. Avian Biol. 47, 312–320. ( 10.1111/jav.00757) [DOI] [Google Scholar]
- 19.Wenzel JW. 1992. Behavioral homology and phylogeny. Annu. Rev. Ecol. Syst. 23, 361–381. ( 10.1146/annurev.es.23.110192.002045) [DOI] [Google Scholar]
- 20.Weber JN, Peterson BK, Hoekstra HE. 2013. Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature 493, 402–405. ( 10.1038/nature11816) [DOI] [PubMed] [Google Scholar]
- 21.Ericson PGP, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson US, Norman JA. 2002. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proc. R. Soc. Lond. B 269, 235–241. ( 10.1098/rspb.2001.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jønsson KA, Fabre PH, Ricklefs RE, Fjeldså J. 2011. Major global radiation of corvoid birds originated in the proto-Papuan archipelago. Proc. Natl Acad. Sci. USA 108, 2328–2333. ( 10.1073/pnas.1018956108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggerbeck M, Fjeldså J, Christidis L, Fabre PH, Jønsson KA. 2014. Resolving deep lineage divergences in core corvoid passerine birds supports a proto-Papuan island origin. Mol. Phylogenet. Evol. 70, 272–285. ( 10.1016/j.ympev.2013.09.027) [DOI] [PubMed] [Google Scholar]
- 24.Gill F, Donsker D (eds). 2016. IOC world bird list (v 6.1). See http://www.worldbirdnames.org/ (accessed April 2016).
- 25.Higgins PJ, Peter JM, Steele WK. 2001. Handbook of Australian, New Zealand and Antarctic birds, vol. 5 Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Higgins PJ, Peter JM. 2002. Handbook of Australian, New Zealand and Antarctic birds, vol. 6 Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Higgins PJ, Peter JM, Cowling S. 2006. Handbook of Australian, New Zealand and Antarctic birds, vol. 7 Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Beruldsen G. 2003. Australian birds, their nests and eggs. Kenmore Hills, QLD: G Beruldsen. [Google Scholar]
- 29.Garnett ST, et al. 2015. Biological, ecological, conservation and legal information for all species and subspecies of Australian bird. Sci. Data 2, 150061 ( 10.1038/sdata.2015.61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udvardy MDF. 1975. A classification of the biogeographical provinces of the world. IUCN occasional paper no. 18. Morges, Switzerland: IUCN.
- 31.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 32.Hugall AF, Stuart-Fox D. 2012. Accelerated speciation in colour-polymorphic birds. Nature 485, 631–634. ( 10.1038/nature11050) [DOI] [PubMed] [Google Scholar]
- 33.Burleigh JG, Kimball RT, Braun EL. 2015. Building the avian tree of life using a large-scale, sparse supermatrix. Mol. Phylogenet. Evol. 84, 53–63. ( 10.1016/j.ympev.2014.12.003) [DOI] [PubMed] [Google Scholar]
- 34.Rubolini D, Liker A, Garamszegi LZ, Møller AP, Saino N. 2015. Using the BirdTree.org website to obtain robust phylogenies for avian comparative studies: a primer. Curr. Zool. 61, 959–965. ( 10.1093/czoolo/61.6.959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabosky DL. 2015. No substitute for real data: a cautionary note on the use of phylogenies from birth–death polytomy resolvers for downstream comparative analyses. Evolution 69, 3207–3216. ( 10.1111/evo.12817) [DOI] [PubMed] [Google Scholar]
- 36.Maddison WP, Maddison DR. 2016. MESQUITE: a modular system for evolutionary analysis (v. 3.04). See http://mesquiteproject.org.
- 37.Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character's effect on speciation and extinction. Syst. Biol. 56, 701–710. ( 10.1080/10635150701607033) [DOI] [PubMed] [Google Scholar]
- 38.Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igić B. 2010. Species selection maintains self-incompatibility. Science 330, 493–495. ( 10.1126/science.1194513) [DOI] [PubMed] [Google Scholar]
- 39.Rabosky DL, Goldberg EE. 2015. Model inadequacy and mistaken inferences of trait-dependent speciation. Syst. Biol. 64, 340–355. ( 10.1093/sysbio/syu131) [DOI] [PubMed] [Google Scholar]
- 40.Irestedt M, Ohlson JI. 2008. The division of the major songbird radiation into Passerida and ‘core Corvoidea’ (Aves: Passeriformes)—the species tree vs. gene trees. Zool. Scr. 37, 305–313. ( 10.1111/j.1463-6409.2007.00321.x) [DOI] [Google Scholar]
- 41.Miller ET, Zanne AE, Ricklefs RE. 2013. Niche conservatism constrains Australian honeyeater assemblages in stressful environments. Ecol. Lett. 16, 1186–1194. ( 10.1111/ele.12156) [DOI] [PubMed] [Google Scholar]
- 42.Frith CB, Frith DW. 2004. The bowerbirds. Oxford, UK: Oxford University Press. [Google Scholar]
- 43.Frith CB, Frith DW. 1990. Nesting biology and relationships of the Lesser Melampitta Melampitta lugubris. Emu 90, 65–73. ( 10.1071/MU9900065) [DOI] [Google Scholar]
- 44.Claramunt S, Cracraft J. 2015. A new time tree reveals Earth history's imprint on the evolution of modern birds. Sci. Adv. 1, e1501005 ( 10.1126/sciadv.1501005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noske R, Bonan A. 2016. Australasian treecreepers (Climacteridae). In Handbook of the birds of the world alive (eds del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E). Barcelona, Spain: Lynx Edicions; See http://www.hbw.com/ (accessed May 2016). [Google Scholar]
- 46.Lima SL. 2009. Predators and the breeding bird: behavioural and reproductive flexibility under the risk of predation. Biol. Rev. 84, 485–513. ( 10.1111/j.1469-185X.2009.00085.x) [DOI] [PubMed] [Google Scholar]
- 47.Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 48.Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. 2014. Female song is widespread and ancestral in songbirds. Nat. Commun. 5, 3379 ( 10.1038/ncomms4379) [DOI] [PubMed] [Google Scholar]
- 49.Leighton GM. 2016. Evolutionary mechanisms maintaining nest construction in avian clades. Avian Biol. Res. 9, 44–51. ( 10.3184/175815516X14500793412915) [DOI] [Google Scholar]
- 50.Price JJ, Lanyon SM. 2002. Reconstructing the evolution of complex bird song in the oropendolas. Evolution 56, 1514–1529. ( 10.1111/j.0014-3820.2002.tb01462.x) [DOI] [PubMed] [Google Scholar]
- 51.Dawkins R. 1982. The extended phenotype. Oxford, UK: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nest data for Australian passerine species (electronic supplementary material, table S1) and world passerine families (electronic supplementary material, table S2), results of BiSSE analyses (electronic supplementary material, table S3), and ancestral state reconstructions of nest characteristics (electronic supplementary material, figure S1 and electronic supplementary material, figure S2) have been uploaded as part of the electronic supplementary material.