Abstract
Vascular diseases span diverse pathology, but frequently arise from aberrant signaling attributed to specific membrane-associated molecules, particularly the Eph-ephrin family. Originally recognized as markers of embryonic vessel identity, Eph receptors and their membrane-associated ligands, ephrins, are now known to have a range of vital functions in vascular physiology. Interactions of Ephs with ephrins at cell-to-cell interfaces promote a variety of cellular responses such as repulsion, adhesion, attraction, and migration, and frequently occur during organ development, including vessel formation. Elaborate coordination of Eph- and ephrin-related signaling among different cell populations is required for proper formation of the embryonic vessel network. There is growing evidence supporting the idea that Eph and ephrin proteins also have postnatal interactions with a number of other membrane-associated signal transduction pathways, coordinating translation of environmental signals into cells. This article provides an overview of membrane-bound signaling mechanisms that define vascular identity in both the embryo and the adult, focusing on Eph- and ephrin-related signaling. We also discuss the role and clinical significance of this signaling system in normal organ development, neoplasms, and vascular pathologies.
Keywords: vascular development, embryo, EphB4, ephrinB2, caveolae, angiosarcoma
Introduction
Blood vessels are one of the first structures formed in the vertebrate embryo. The enlarging size of the successfully developing embryo outgrows the capacity for simple diffusion to provide nutrient and oxygen transfer that was previously sufficient during the first several days of embryonic life. Therefore, functionally patterned vascular networks are critical for hematogenous transfer of nutrients in the developing embryo, and remain essential for maintenance of tissues and organ systems into adulthood. The vascular networks consist of arterial, venous, and lymphatic systems. Arteries and veins have traditionally been defined by the direction, pressure, and oxygen tension of blood flow within them, with arteries transmitting high-pressure and high-oxygen blood away from the heart and veins transmitting low-pressure and low-oxygen blood back. These functional characteristics are reflected in their morphological differences. Arteries have a thicker wall of smooth muscle and elastin layers between the endothelium and adventitia; in contrast, veins are thinner with fewer smooth muscle and elastin layers. The lymphatic vasculature, the third component of the vascular system, is essential for maintaining interstitial fluid homeostasis, immune function, and for the absorption of dietary fat (Stacker et al., 2014). Originally formed from the embryonic veins (Yang and Oliver, 2014), the mammalian lymphatic system consists of the two distinct types of vessels: lymphatic capillaries and lymphatic collecting vessels (Jurisic and Detmar, 2009). Lymphatic capillaries are thin-walled vessels with a single layer of overlapping endothelial cells, blindly ending within tissue spaces to absorb interstitial fluid. Lymphatic collecting vessels are larger caliber vessels with contractile properties, supported by smooth muscle cells (SMCs) and containing intraluminal valves to facilitate the transport of lymph against hydrostatic pressure to the thoracic duct (Baluk et al., 2007; Lynch et al., 2007; Scallan et al., 2013; Yang and Oliver, 2014).
It is now known that arteries and veins also contain unique genetic markers that not only mark but determine cell fate. Both arterial and venous endothelial cells are genetically predetermined from the early stage of angiogenesis, as early as embryonic day 8.5, even prior to functional blood flow during embryonic development (Andres et al., 1994; Bennett et al., 1995; Bergemann et al., 1995; Wang et al., 1998). The endothelial cells in blood vessel sprouts were found to differentiate into arteries or veins, in concert with their expression of several tyrosine kinases acting as distinct cellular determinants; in particular, the intermembrane interaction between ephrinB2 ligands on arteries and EphB4 receptors on veins is the critical and sufficient determinant of embryonic vasculature (Wang et al., 1998). The expansion of vascular networks with functional arterial and venous systems is also directed by the interaction between EphB4 receptors and its ephrinB2 ligand (Fig. 1A). On the other hand, lymphatic endothelial cell progenitors are initially specified in the embryonic veins (Yang and Oliver, 2014). These progenitors continue to differentiate and mature as they bud from the veins to produce scattered primitive lymph sacs, from which most of the lymphatic vasculature is derived (Yang and Oliver, 2014). EphrinB2 has been identified as an essential regulator of collecting lymphatic vessels and lymphatic valves (Makinen et al., 2005).
FIGURE 1.
Schematic showing (A) details of EphB4 and ephrinB2 structures, as well as (B) bidirectional signaling induced by EphB4-ephrinB2 binding. Panel (C) depicts pathways leading to selective EphB4 or ephrinB2 expression guiding arterial-venous differentiation. PBM, PDZ binding motif; SH2, SRC homology 2; RBD, receptor-binding domain; LBD, ligand-binding domain; CRD, cysteine-rich domain; Sushi, short consensus repeat (SCR); EGF, epithelial growth factor; FNIII, fibronectin III; SAM, sterile alpha motif (Pasquale, 2008; Boyd et al., 2014).
The erythropoietin-producing hepatocellular (Eph) receptors are the largest of the 14 subfamilies of receptor tyrosine kinases (RTK), and are stimulated by the ephrin family of membrane-associated ligands. Eph receptors are expressed in all germ layers, and play a highly complex and critical role for the embryonic development of various organs in vertebrates. In addition to vascular development, Ephs and ephrins are essential for axon guidance, cell migration and segregation, tissue boundary formation, and topographic mapping (Kullander and Klein, 2002; Klein, 2012). Ephrins can be divided into two subclasses depending on their structural characteristics: ephrinA ligands are tethered to the cell surface via a glycosylphosphatidylinositol (GPI)-anchor, whereas ephrinB ligands are inserted into the plasma membrane via a transmembrane region followed by a conserved cytoplasmic domain (Adams et al., 1999). Correspondingly, Ephs can be divided into EphA and EphB subclasses, based on their sequence similarities and binding affinity to ephrins (Gale et al., 1996).
Interactions between Ephs and ephrins promote various cell responses, including repulsion, attraction, adhesion, and migration. The repulsive response is important for cell guidance and sorting (Pitulescu and Adams, 2014). Hence this repulsion function of Eph and ephrin signaling is thought to enable boundary formation of organs in the developing embryo. Migrating cells expressing Eph receptors turn away from cells expressing particular ephrin ligands (Drescher et al., 1995; Nakamoto et al., 1996), although in some contexts, Eph and ephrin signaling can promote adhesion of cells (Holmberg et al., 2000; Dravis et al., 2004). For example, topographic mapping, the mechanism by which axon terminals organize themselves onto a particular target area, is regulated by either repulsive or adhesive/attractive forces through Eph-ephrin signaling.
Since the Eph receptors and ephrin ligands are tethered to the plasma membrane, it is likely that the Eph-ephrin system functions during cell-to-cell interactions, rather than during long-range communications (Davis et al., 1994; Orioli and Klein, 1997; Adams et al., 1999). In this context, ephrin molecules are frequently found in a clustered state, thereby achieving a stronger stimulus for Eph receptor activation (Davis et al., 1994) (Fig. 1B). Ephrin stimulation of EphB4 receptors through cell-cell contact induces receptor autophosphorylation, initiating propagation of a diverse signaling cascade below the membrane, characteristic of tyrosine kinase receptors (Bae and Schlessinger, 2010). The downstream signaling of Eph receptors is complicated, since there are different signaling proteins containing a Src homology 2 domain (SH2) that can interact with two juxtamembrane tyrosine residues in the Eph cytoplasmic domain (Kalo and Pasquale, 1999). PI3K (Pandey et al., 1994), Src family kinase (Zisch et al., 1998), SLAP (Pandey et al., 1995), Grb2/10 (Stein et al., 1996), and PLCγ are all known to be SH2-containing mediators of Eph signaling.
Remarkably, Eph-ephrin signaling can be bidirectional. EphrinB2 acts both as a ligand and simultaneously as a receptor for EphB4; thus, binding and clustering of ephrins to the Eph receptors can lead to activation of intracellular signaling via both the Eph receptor (canonical forward signaling), as well as the ephrin ligand (reverse signaling) (Holland et al., 1996; Bruckner et al., 1997; Kullander and Klein, 2002) (Fig. 1B). Generally, forward signaling via the Eph receptor generates a repulsive cell response away from the ephrin-expressing cell, while reverse signaling via ephrin generates an adhesive response (Kao et al., 2012). These reciprocal signaling pathways between two types of vessels are thought to be critical for embryonic angiogenic remodeling (Wang et al., 1998; Yancopoulos et al., 1998).
Although Ephs and ephrins were first recognized as pivotal regulators of embryonic vascular development, it is now known ephrinB2 and EphB4 interactions remain essential for vessel remodeling and plasticity in adult vessels (Gale et al., 2001; Kullander and Klein, 2002; Adams, 2003; Foo et al., 2006; Swift and Weinstein, 2009; Muto et al., 2010). For example, the process of vein graft adaptation to the postsurgical arterial environment is characterized by loss of the venous marker EphB4, but without gaining the arterial marker ephrinB2, suggesting the plasticity of vessel identity, even in aged adult vessels (Kudo et al., 2007). Eph and ephrin expression patterns and the consequences for vessel remodeling may be fundamentally different in other clinically relevant changes in response to hemodynamic flow, such as the disturbed flow pattern found in arteriovenous fistulae. While these changes have not yet been reported, it is possible that these markers of vessel identity continue to have a critical role in the adult vascular system, with potential translational applications for human vascular diseases and therapies. While there is a basic understanding of some of these signaling pathways, considerable gaps remain in our knowledge of the mechanisms underlying vascular pathophysiology.
We review recent progress in our understanding of membrane-mediated regulation of arterial and venous identity in the developing, regenerative, and remodeling adult vasculature. Lymphatic vessels have recently been reviewed elsewhere (Stacker et al., 2014; Yang and Oliver, 2014). We focus on evidence that links Ephs and ephrins to functional membrane signaling and intracellular signal transduction, discussing the significance of these signals in guiding adaptive remodeling of the vascular system, the physiology of placenta and bone development, and some examples of tumorigenesis. We apologize in advance to our many colleagues whose work we could not include or may have inadvertently overlooked.
Arterial and Venous Identity from Development to Adulthood
Vasculogenesis is the process of de novo blood vessel formation that first occurs during the earliest stage of embryogenesis. Vasculogenesis begins before the onset of the heartbeat; hemangioblast precursors from the mesoderm migrate, aggregate as blood islands, and differentiate into endothelial cells to form the first blood vessels (Risau and Flamme, 1995). Factors such as vascular endothelial growth factors (VEGF), angiopoietins, and basic fibroblast growth factor (bFGF), and receptors such as VEGF receptors, neuropilin1, and tie2, are thought to be primary mediators of vasculogenesis (Moyon et al., 2001). Following vasculogenesis, arteries and veins differentiate to form a functional circulatory system. This secondary process is characterized by sprouting of new vessels from pre-existing ones, termed angiogenesis. These sprouts then fuse with other sprouts or pre-existing vessels to form loops and hierarchical networks, leading to vascular beds that sustain functions of vital tissues and organs. In contrast to vasculogenesis that mainly occurs during embryonic development, angiogenesis is typically the mechanism for generating new vessels during adulthood. These two mechanisms are also distinct from arteriogenesis, the formation and maturation of collateral arteries to improve flow to hypoxic areas after occlusion of an arterial trunk in adults (Carmeliet, 2000; Simons and Eichmann, 2015).
Distinct identities of arteries and veins have been studied in the context of conserved developmental signaling pathways, as well as the contribution of blood flow and extracellular matrix. Here we discuss key signals that control vascular identities and remodeling with an emphasis on Eph and ephrin signaling.
Upstream Eph and Ephrin Signaling
SONIC HEDGEHOG-VEGF-DLL-NOTCH SIGNALING
Sonic hedgehog (Shh) is a transcription factor originally discovered as a morphogen that contributes to early patterning in the developing embryo, regulating epithelial-mesenchymal interactions (Echelard et al., 1993; Chiang et al., 1996). Later, several studies revealed an important role for Shh during blood vessel development; embryos lacking Shh activity fail to show arterial differentiation (Lawson et al., 2001). In arterial fated endothelial cells, Shh induces expression of VEGF (Lawson et al., 2002). Embryos lacking Shh activity fail to express VEGF within their somites, and exogenous addition of VEGF can rescue vascular ephrinB2 expression. Shh also promotes adult angiogenesis by producing a combination of angiogenic factors, inducing VEGF as well as the angiopoietins Ang1 and Ang2 (Pola et al., 2001). Taken together, these observations suggest that Shh-VEGF signaling is responsible for inducing arterial differentiation of endothelial cell progenitors in embryogenesis as well as in adulthood.
The VEGF family members are critical regulators of vascular development during embryogenesis and strongly activate their receptors on endothelial cells to promote the growth of blood vessels and vascular networks. Five subtypes of VEGF ligands bind in an overlapping pattern to three RTKs (VEGFR1-3), as well as to co-receptors such as the neuropilins (Olsson et al., 2006). VEGFR1 regulates monocyte and macrophage migration while VEGFR3 regulates lymphangiogenesis; VEGFR2 is implicated in many aspects of endothelial cell biology, including signaling embryonic vessel formation and regulating vascular permeability (Olsson et al., 2006).
The role of VEGF-Dll-Notch signaling in promoting differentiation of the arterial endothelium is well described (Lawson et al., 2001, 2002; Lanner et al., 2007). Binding of VEGF to its heterodimeric receptor stimulates the Dll-Notch pathway in the endothelium (Lawson et al., 2001, 2002). Graded signaling by VEGF can affect downstream expression of both ephrinB2 and EphB4 (Lanner et al., 2007), highlighting the role of Shh-VEGF as a master signal in vascular differentiation.
VEGF signaling also regulates endocytosis, another critical component of molecular signaling. In the endothelium, ephrinB2 is required for clathrin-mediated endocytosis of VEGFR2, enabling signaling by its ligand VEGF (Pitulescu and Adams, 2014). Conversely, ephrinB2 antagonizes clathrin-mediated endocytosis of platelet-derived growth factor (PDGF) receptor β in vascular SMCs, balancing PDGF activation by different signal transduction pathways (Pitulescu and Adams, 2014).
The Dll-Notch signaling system is a highly conserved mechanism used by multicellular organisms to control cell fate through cell-to-cell interactions (Artavanis-Tsakonas et al., 1999). In vertebrates, there are four heterodimeric, transmembrane receptors (Notch1-4) and 5 ligands (Jagged1, Jagged2, Dll1, Dll3, and Dll4). Since most Notch ligands are also transmembrane proteins, the receptors are normally only triggered from direct contact of neighboring cells.
The Notch signaling pathway is important in driving arterial endothelial cell differentiation, with Dll4 ligand being one of the first identified arterial-specific markers (Shutter et al., 2000). In the absence of Dll4 or Notch signaling, vascular endothelial cells show a hyperproliferative phenotype leading to vascular malformations (Hellström et al., 2007; Siekmann et al., 2013). Hence, venous cell fate was classically thought to be the default pathway of vascular development via lack of activation of Notch signaling; later it was suggested that the venous pathway is not a default pathway, but rather it is under active control of chicken ovalbumin upstream promoter transcription factor 2 (COUP-TFII), via suppression of Notch signaling (You et al., 2005). Lymphatic fate is also regulated by COUP-TFII and the Notch pathway. Down-regulation of Notch activity leads to increased numbers of lymphatic endothelial cell progenitors, suggesting that Notch signaling is a negative regulator of lymphatic endothelial cell specification (Murtomaki et al., 2013).
Numerous transgenic studies using model organisms such as zebrafish are revealing the spatiotemporal detail of when and where Notch activation occurs during vascular endothelial cell differentiation. Of note, while Notch activation is maintained in arterial fated endothelial cells during embryogenesis (Quillien et al., 2014), some Notch activated cells then down-regulate Notch signaling and contribute to venous remodeling. Thus Notch seems to be acting during initiation and maintenance of arterial identity during embryogenesis.
Dll-Notch continues to stimulate the arterial fate pathway in embryonic vascular cells by causing increased ephrinB2 expression with simultaneous suppression of EphB4 expression; thus, Dll-Notch prevents acquisition of a venous fate (Siekmann and Lawson, 2007). During vein graft adaptation to the arterial environment in rats, both Dll4 and Notch4 expression are down-regulated in an aged background, suggesting the Dll4-Notch pathway may also be active in adult venous remodeling (Kondo et al., 2011).
COUP-TFII
COUP-TFII is a transcriptional factor critical for establishing venous identity during embryonic vascular development. COUP-TFII is also known as NR2F2 (nuclear receptor subfamily 2, group F, member 2), an orphan member of the steroid receptor superfamily; COUP-TFII has various expression in different tissues and serves a variety of functions in organ development, tumor biology, stem cell differentiation, and lipid and glucose metabolism (Takamoto et al., 2005; Xu et al., 2008; Hu et al., 2013; Qin et al., 2013). Of interest, COUP-TFII is highly expressed in the endothelium of embryonic and adult veins, but not in arterial endothelial cells (You et al., 2005; Cui et al., 2015). Primary venous specification appears to be under control of this transcription factor, via suppression of Notch signaling (You et al., 2005). A working model that maintains venous identity is proposed: during embryogenesis, COUP-TFII in venous endothelium down-regulates VEGF and its co-receptor neuropilin1, preventing Notch pathway activation, thereby relieving suppression of EphB4 expression, without inducing expression of ephrinB2 (You et al., 2005; Fancher et al., 2008) (Fig. 1C).
COUP-TFII has a broader role in regulating some patho-physiologic functions in adult blood vessels, beyond its role of arterial venous specification in embryonic development. Suppression of COUP-TFII in venous endothelial cells facilitated phenotype switching toward proatherogenic and susceptibility to endothelial-to-mesenchymal transition, leading to subsequent osteogenesis and calcium deposition; up-regulation of inflammation and down-regulation of antithrombotic signals were proposed as a mechanism (Cui et al., 2015). As a member of the nuclear receptor family, COUP-TFII activity can be regulated by small molecules (Kruse et al., 2008), which may be attractive to pharmacological targeting and translational applications to reduce neointimal hyperplasia and atherosclerosis.
Functions of EphB4 and EphrinB2 During Vascular Development
BLOOD VESSEL SPROUTING
Sprouting blood vessels consist of two different cell populations with distinct behaviors: tip cells and stalk cells. The role of tip cells in angiogenesis was first described in quail embryos (Kurz et al., 1996). Endothelial tip cells navigate through avascular tissues, followed by stalk cells (Siekmann et al., 2013). While following the tip cells, stalk cells form the base of the sprout and maintain the connection to the parental vessel, ultimately leading to a hierarchical network that allows efficient tissue perfusion (Geudens and Gerhardt, 2011). The specification of tip and stalk cells is under control of Dll-Notch signaling (Potente et al., 2011). One recent study showed neuropilin-1 is a critical downstream effector of Notch in tip/stalk cell specification in angiogenesis (Aspalter et al., 2015).
ANGIOGENIC CELL MIGRATION AND AXON GUIDANCE
The graded extracellular distribution of VEGF-A orchestrates tip and stalk cell behavior (Hellström et al., 2007). VEGF-A stimulates endothelial cells to differentiate into tip cells. However, downstream Dll-Notch signaling functions to limit the explorative behavior of tip cells. Behaviors of tip cells during angiogenesis have similarities with those of axonal growth cones during neurite outgrowth, and they share a number of guidance cues, such as Semaphorin-Plexin, Netrin-Unc, and Slit-Robo ligand-receptor pairs (Weinstein, 2005; Larrivee et al., 2009; Siekmann et al., 2013). Recently, time-lapse imaging and genetic lineage tracing showed specific migratory behaviors of tip cells during regenerating angiogenesis in arteries (Xu et al., 2014). Arterial-fated tip cells derive from venous endothelial cells, and change their migration direction backwards to the vascular plexus. This behavior is mediated by chemokine-receptor Cxcr4a-Cxcl12a function (Xu et al., 2014).
ARTERIAL/VENOUS SEGREGATION
Eph and ephrin proteins are expressed reciprocally in many tissues during development. Studies of the hindbrain have shown that Eph-ephrin signaling contributes to boundary formation in organ development (Fraser et al., 1990; Guthrie et al., 1993; Xu et al., 1995, 1999). These functions are mediated by regulation of the actin cytoskeleton, cadherin function, and integrin-mediated adhesion (Batlle and Wilkinson, 2012). Eph-ephrin control of actin cytoskeleton dynamics leads to changes in cellular shape or repulsive responses (Klein, 2012). Interestingly, complementary expression of ephrinB and EphB is sufficient to segregate intermingled cells, maintaining sharp borders by increasing adhesion within specific areas and by repulsing across borders (Batlle and Wilkinson, 2012).
FORMATION OF THE VASCULATURE REQUIRES EPH-EPHRIN SIGNALING
EphB4-ephrinB2 signaling is required for the formation of the first two axial vessels, the dorsal aorta, and the cardinal vein. In the zebrafish embryo, angioblasts initially form a single precursor vessel that expresses both ephrinB2 and EphB4. Ventral migration of EphB4-positive endothelial cells leads to the separation of the dorsal aorta from the cardinal vein by repulsion from the ephrinB2-positive cells (Herbert et al., 2009). In mice, the cardinal vein forms by sprouting of EphB4-positive endothelial cells from an early dorsal aorta that contains ephrinB2-positive, EphB4-positive, and uncommitted precursor cells (Pitulescu and Adams, 2014).
EphrinB2 and EphB4 are expressed reciprocally in the yolk sac arteries and veins of mouse embryos (Wang et al., 1998). Interestingly, the arterial marker ephrinB2 is also expressed in a subset of veins in neonatal human tissue and pathologic adult arterialization (Diehl et al., 2005). Consistently, in full-term human umbilical cords, ephrinB2 expression is observed in veins as well as in arteries, together with an additional arterial marker neuropilin1; the venous marker EphB4 expression is observed in veins, but less so in arteries (Fig. 2).
FIGURE 2.
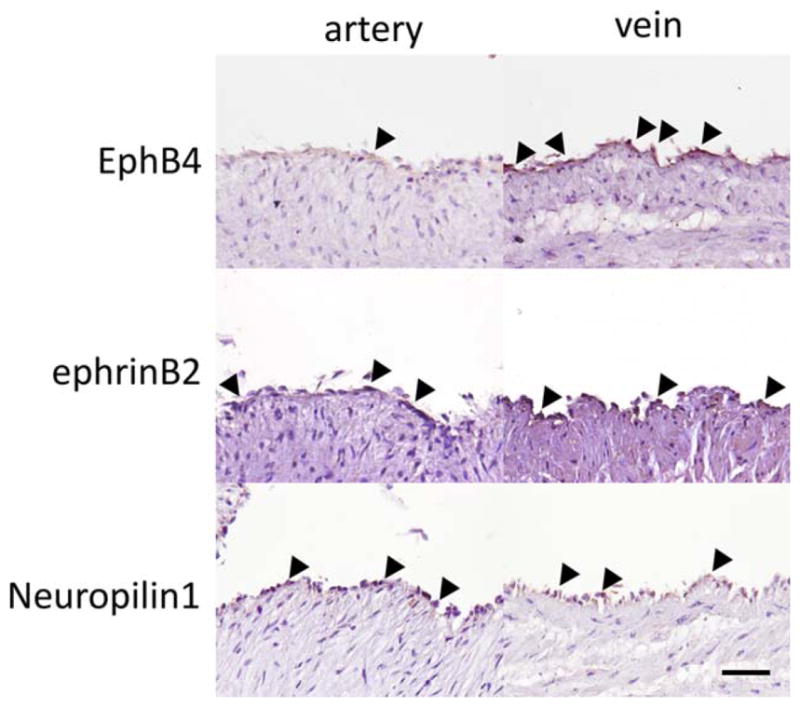
Representative photomicrographs showing expression of the venous identity marker EphB4 and the arterial identity markers ephrinB2 and neuro-pilin1 in full term human umbilical cord artery and vein. Black arrowheads indicate positive signals. Scale bar 50 μm.
Genetic deletion of EphB4 or ephrinB2 results in several significant cardiovascular defects in mice, including disruption of yolk sac angiogenesis (Wang et al., 1998). Furthermore, in vivo data show that deletion of the cytoplasmic tail of ephrinB2 caused similar defects with the phenotype of mice that lack the entire protein, suggesting that the ephrinB2 cytoplasmic domain is required for remodeling into a hierarchical vascular system (Adams et al., 2001). Thus ephrinB2 reverse signaling may be required for remodeling of the embryonic vasculature.
EphB4 and EphrinB2-Associated Signaling
Eph and ephrin proteins interact with a number of other ligand/receptor molecules on and below the cell membrane, regulating translation of environmental signals to mediate organ development. This regulatory system functions in concert with others; for example, the post-natal vessel endothelium also releases several soluble cytokines, including regulators of extracellular matrix remodeling and vasoactive molecules that regulate wall remodeling. These systems are integrated to regulate vessel adaptation to mechanical stresses, such as flow, pressure, and tension (Rothuizen et al., 2013; Lu et al., 2014). Here we discuss examples of signaling pathways that mediate EphB4 or ephrinB2 functions (Fig. 3).
FIGURE 3.
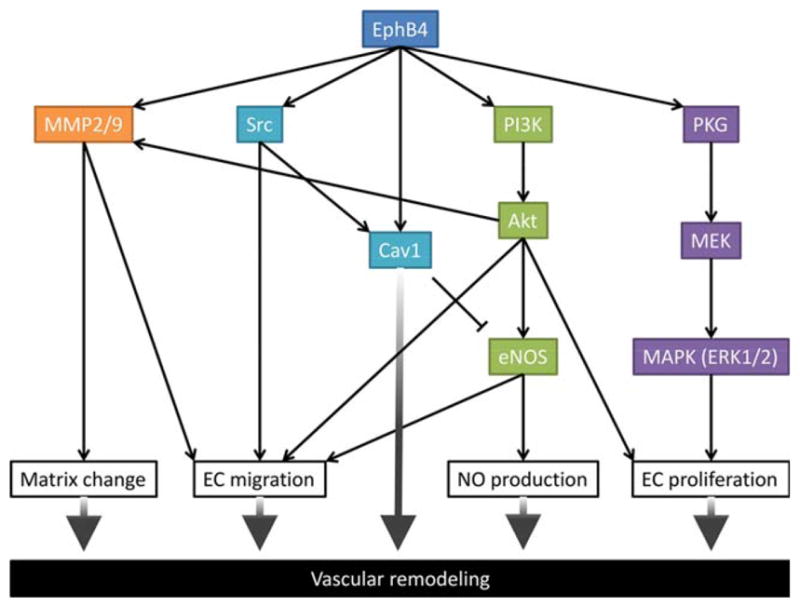
Schematic showing signaling pathways downstream of EphB4 regulate vascular remodeling. MMP2/9, matrix metalloproteinase 2/9; Src, proto-oncogene tyrosine-kinase kinase; Cav1, caveolin1; PI3K, phosphatidylinositol-3-kinase, eNOS, endothelial nitric oxide synthase; PKG, cGMP-dependent protein kinase; MEK, mitogen-activated protein kinase; MAPK, mitogen-activated protein kinase; Erk, extracellular signal-regulated kinases.
CAVEOLIN1
Caveolae and caveolin1
Caveolae are distinct flask-shaped invaginated structures with a diameter of 60 to 80 nm that are located along the plasma membrane (Bruns and Palade, 1968). These unique nanostructures are recognized in the surface of many cell types, including endothelial cells, fibroblasts, and adipocytes. Caveolae are cholesterol- and sphingolipid-rich membrane microdomains that serve as a signaling platform to facilitate the temporal and spatial localization of various signal transduction events, including endocytosis, lipid regulation, and vessel remodeling. Of note, various signaling molecules, including Eph receptors, seem to enhance their downstream signaling by localizing within caveolae (Rivera et al., 2009).
Caveolins were identified as the major component of caveolae in the 1990s (Rothberg et al., 1992; Tang et al., 1996). The N and C terminals of caveolin reside in the cytoplasm, whereas a 33-amino acid hydrophilic segment is predicted to insert into plasma membranes, resulting in its putative hairpin shape (Dupree et al., 1993). Among the three isoforms of caveolin (Cav1, Cav2, and Cav3), Cav1 is the major scaffolding protein of caveolae and is necessary for caveolae assembly (Drab et al., 2001). Other components, such as cavins and Pacsin2, have also been recognized as essential for the shape and function of caveolae (Aboulaich et al., 2004; Hill et al., 2008; Hansen et al., 2011; Walser et al., 2012).
The Cav1 isoform is particularly abundant in endothelial cells and has been the most extensively studied amongst the three isoforms. Cav1 regulates various endothelial functions, including transcytosis, permeability, vascular tone, and angiogenesis (Frank et al., 2003; Woodman et al., 2003). In endothelial cells, Cav1 is present throughout the vasculature, while Cav3 is found in arterial but not in venous vasculature (Segal et al., 1999), showing a distinct distribution of caveolin isoforms.
Caveolae as a mechano-sensor at membrane
In general, caveolae are abundant in cells subjected to mechanical stress, such as muscle cells, fibroblasts, and endothelial cells (Nassoy and Lamaze, 2012; Parton and del Pozo, 2013). Cells respond to acute mechanical stresses by disassembly and reassembly of caveolae (Sinha et al., 2011). Trafficking and organization of caveolae in these processes are tightly regulated by stress-fiber regulators, implying a functional link between caveolae and stress fibers (Echarri and Del Pozo, 2015).
In endothelial cells, Cav1 interacts with a large number of signaling molecules that are involved in shear stress-mediated activation including G proteins, tyrosine kinases, GTPases, eNOS, and some components of the MAPK pathway (Smart et al., 1999; Gratton et al., 2004). These observations suggest that Cav1 and/or caveolae serve as flow-activated mechano-sensors in blood vessels. In particular, caveolae sense flow at the luminal surface of endothelial cells; Cav1 knockout mice show impaired shear stress regulation of arterial diameter, and these effects are restored by re-expression of endothelial Cav1 (Yu et al., 2006). This clear genetic evidence supports the important role of Cav1 in mechano-transduction of hemodynamic forces in blood vessels. Interestingly, the number of caveolae at the cell surface increases in flow-adapted endothelial cells; however, prolonged exposure to laminar shear stress alters the distribution but not the expression of Cav1 within the endothelium in vitro (Rizzo et al., 2003), suggesting spatiotemporal complexity of Cav1 expression in response to mechanical stress.
This evidence suggests that endothelial caveolae are an efficient means to modulate cell surface signaling that arise under conditions of altered blood flow. However, since Cav1 has other functions than simply a scaffold within caveolae (Pol et al., 2004), interpreting results from Cav1 loss-of-function studies should be made with caution.
Cav1 tyrosine 14 phosphorylation
Cav1 was originally identified as a major tyrosine-phosphorylated substrate of Src kinase, since tyrosine phosphorylation of Cav1 on Y14 is associated with sarcoma virus transformation (Glenney, 1989; Glenney and Soppet, 1992). In cancer cells, tyrosine phosphorylation of Cav1 induces caveolae biogenesis via actin-dependent mechanotransduction, together with inactivation of the Cav1-suppressing transcription factor early growth response (Egr)–1 (Joshi et al., 2012). Phosphorylation of Cav1 also occurs in response to growth factor stimulation and integrin-mediated mechanotransduction (Mastick et al., 1995; Kim et al., 2000; Lee et al., 2000; Fielding et al., 2004; Radel and Rizzo, 2005). Src-mediated phosphorylation of Cav1 is indispensable in stretch-induced epidermal growth factor receptor (EGFR) transactivation that leads to Akt phosphorylation (Zhang et al., 2007). These data imply that Cav1 phosphorylation has an important role in cell adaptation when subjected to mechanical stress by blood flow or stretch.
Interaction between Cav1 and Eph receptors
Cav1 interacts with several receptor-tyrosine kinases. The family of Eph RTKs translocates to, or resides within, caveolae, in close physical proximity to Cav1 (Couet et al., 1997; Yamamoto et al., 1998; Lajoie et al., 2007). More specifically, EphB1 receptors are localized in caveolae, and directly interact with Cav1 upon ligand stimulation; Cav1 mediates EphB1 receptor downstream ERK signaling (Vihanto et al., 2006). Furthermore, EphA2 also interacts with Cav1.
In wild-type isolated endothelial cells, stimulation of EphB4 signaling results in increased Cav1 phosphorylation, endothelial cell migration, and nitric oxide (NO) production (Muto et al., 2011). However, these effects were reduced in endothelial cells derived from heterozygous EphB4 mice. This observation shows that Cav1 is a downstream mediator of EphB4 signaling, regulating at least some endothelial cell functions in vitro. In a mouse vein graft model, elimination of Cav1 abolishes vein graft thinning in response to EphB4 stimulation, suggesting that Cav1 is a critical mediator of EphB4 function in vivo (Muto et al., 2011). As such, Cav1 is an important downstream mediator of EphB4 signaling both in vitro and in vivo.
Interaction with eNOS
The structure of the eNOS protein facilitates dual acylation targeting to the cytoplasmic aspect of the Golgi complex and to plasmalemmal caveolae (Sessa et al., 1995; Garcia-Cardena et al., 1996). Co-immunoprecipitation studies show that nearly all the eNOS in endothelial cells is associated with Cav1 (Feron et al., 1996). On the caveolae side, the Cav1 scaffolding domain serves as an endogenous negative regulator of eNOS function (Bernatchez et al., 2011). Specifically, phenylalanine 92 (F92) is critical for this inhibitory action of Cav1 on eNOS. The activity of eNOS is suppressed under resting conditions in a reciprocal manner via its interaction with Cav1, leading to lower basal NO production (García-Cardeña et al., 1997; Ju et al., 1997; Michel et al., 1997). Since targeted interference with the inhibitory actions of Cav1 enhances NO release (Bernatchez et al., 2011), this specific strategy may be valuable for cardiovascular diseases characterized by NO deficiency.
Interaction with TGF-β signaling
Cav1 is also an important negative regulator of TGF-β signaling (Razani et al., 2001). TGF-β signaling typically plays an important role in cellular differentiation, with the attainment of a terminal phenotype frequently depending on cessation of TGF-β signaling (Moses and Serra, 1996). Caveolins, including Cav1, are frequently expressed in terminally differentiated cells in TGF-β-responsive lineages (Scherer et al., 1997). Therefore, Cav1’s suppressive regulation of TGF-β signaling could be an important mechanism for the controlled progression of developmental events of organs, including vessels (Razani et al., 2001).
TGF-β has also been implicated in pathologic processes in the adult vasculature. TGF-β is a potent stimulator of extracellular matrix deposition during early stages of vein graft adaptation; enhanced TGF-β signaling promotes progressive fibrotic neointimal hyperplasia expansion (Jiang et al., 2009). TGF-β signaling is also a principal pathway regulating endothelial-mesenchymal transition, an important component of vein graft remodeling in mice and possibly humans (Cooley et al., 2014). However, it is not well known whether Cav1 attenuates TGF-β-responsive pathological vessel remodeling.
Phenotype of Cav1−/− mice
Genetic deletion of Cav1 leads to complete loss of caveolae in mice. The phenotype of these mice includes cardiac and pulmonary abnormalities, derangements of lipid metabolism, and eNOS dysfunction (Drab et al., 2001; Razani and Lisanti, 2001), signifying the importance of Cav1 function in adult life. However, it is intriguing that complete loss of caveolae is not a lethal phenotype. Cav1 knockout mice are even fertile and appear grossly normal. These observations suggest that if compensatory pathways exist, they are not within caveolae, since Cav1 is required for caveolae assembly (Parton, 2001). A recent study reporting genetic deletion of Cav1 in rodents showed a remarkable prevention of abdominal aortic aneurysm development in an Ang II-BAPN co-infusion model (Takayanagi et al., 2014), adding evidence that supports active participation of Cav1 in adult vascular pathology.
AKT
The PI3K-Akt pathway has critical roles in regulating diverse cellular functions, such as cell survival, proliferation, and metabolism. PI3K is a potential Eph receptor-binding partner, since the p85 subunit possesses a C-terminal SH2 domain that is able to interact with Eph receptors; in particular, PI3K binds to the EphA2 receptor (Pandey et al., 1994).
Steinle et al. (2002) reported that stimulation of EphB4 with the specific ligand ephrinB2/Fc augments migration and proliferation of human microvascular endothelial cells. These effects were inhibited in the presence of PI3K or Akt-inhibitors, suggesting the PI3K-Akt pathway plays one of the central roles in EphB4 signaling in endothelial cells and vascular remodeling.
ENOS
eNOS-derived NO plays important roles in many physiologic and pathologic cardiovascular events (Forstermann and Sessa, 2012). Many studies have shown that EphB4 regulates NO release in endothelial cells. With ephrinB2/Fc stimulation, eNOS phosphorylation and NO production are increased in human endothelial cells (Wong et al., 2014), while mouse endothelial cells derived from heterozygous EphB4 mice have less NO release compared with wild type (EphB4+/+) endothelial cells (Jadlowiec et al., 2013).
We have shown that eNOS is likely to be an essential downstream mediator of EphB4 signaling that occurs during venous adaptation to the arterial environment (Wang et al., 2015). Stimulation of EphB4 with monomeric eph-rinB2/Fc or clustered ephrinB2/Fc resulted in increased eNOS phosphorylation, NO production, and cell migration in vitro; these effects were abolished in the presence of an eNOS inhibitor. These data suggest that EphB4 regulates endothelial cell functions by eNOS phosphorylation. In a mouse vein graft model, loss of EphB4 during vein graft adaptation was associated with increased eNOS activity and adaptive vein graft wall thickening (Wang et al., 2015).
Unanswered questions include the seemingly discordant observations that EphB4 stimulates eNOS phosphorylation in vitro, whereas diminished EphB4 is associated with increased eNOS activity in vein grafts in vivo. It is likely that there are diverse mediators involved in regulating the EphB4-eNOS pathway in vivo, which entails a more complex mix of different cell populations and interactions, compared with isolated endothelial cells in vitro. For example, it is known that eNOS activity leads to NO production, which in turn is able to induce Src activation. Since Src stimulates Cav1 phosphorylation, increased eNOS-Cav1 interaction negatively regulates eNOS phosphorylation (Chen et al., 2012). Another potential mediator is Ephexin (Eph-interacting exchange protein), a guanine nucleotide exchange factor for Rho GTPases that was identified as an Eph-receptor interacting protein with a yeast two-hybrid screen assay. Eph receptors modulate Ephexin activity and lead to Rho kinase activation (Shamah et al., 2001). However, Rho kinase can directly suppress eNOS activity (Sugimoto et al., 2007), or can negatively regulate eNOS activity via Akt (Ming et al., 2002). Although there is much left to investigate, eNOS plays an important role as an effector of EphB4 signaling, highlighting the EphB4-eNOS pathway as a potential attractive target for therapeutic manipulation of vessel remodeling.
ERK1/2
The MAPK ERK signaling pathway is downstream of VEGF and is associated with cell proliferation and migration. ERK is a downstream mediator of EphB4, since ephrinB2/Fc stimulation in human microvascular endothelial cells induces ERK1/2 phosphorylation (Steinle et al., 2002). However, it is also known that EphB4 transduces the ephrinB2 signal through different downstream effector molecules to positively or negatively regulate the ERK pathway in different cell types, reflecting the complexity of EphB4 signaling (Xiao et al., 2012). ERK mediates EphB4-regulated endothelial proliferation; however, this activation of MAPK is likely independent of Ras, since Ras inhibition does not reduce ephrinB2/Fc-induced cell proliferation (Steinle et al., 2002). In addition, EphB4-dependent promotion of endothelial cell migration is not attenuated by an ERK inhibitor, suggesting that ERK is not involved in EphB4-mediated migration (Steinle et al., 2002).
It is noteworthy that the ERK signaling pathway is part of the EphB4 signal transduction pathway not only in endothelial cells, but also in hepatic stellate cells. EphB4 stimulates VEGF production and sinusoidal endothelial cell recruitment via the ERK pathway, and this effect is Akt-independent (Das et al., 2010). Similar pathways may exist in different cell types, e.g., EphB4 activation results in inhibition of the Ras-MAPK-ERK pathway in human vascular endothelial cells, whereas it causes activation of the same pathway in breast cancer cells (Xiao et al., 2012).
OTHERS
Many Src family kinase family members are promising downstream candidates of Eph signaling in the nervous system. Activation of EphB4 by an exogenous ligand in vascular endothelial cells can induce Src phosphorylation. Src is responsible for EphB4-regulated endothelial cell migration, but not proliferation (Steinle et al., 2002).
EphB4-mediated regulation of endothelial cell migration also involves MMP2 and MMP9. Since these MMPs degrade collagen within the basement membrane, MMP2/9 activation is likely to enhance endothelial cell migration (Steinle et al., 2002). EphB4 may regulate MMP2/9 phos-phorylation via an Akt signaling pathway, since EphB4 can activate Akt, and Akt has been correlated with activation of MMP2/9 (Kim et al., 2001; Park et al., 2001).
Pericyte Dynamics During Vascular Development
In addition to endothelial cells, pericytes and vascular SMC are essential components of the vascular system. Unlike endothelial cells, these mural cells are of mesenchymal origin. Pericytes are periendothelial support cells that elongate around the endothelial cells and are functionally involved in stabilization and maturation of sprouting vessels during angiogenesis (Stapor et al., 2014). They were described in 1873 by the French physiologist Charles Marie-Benjamin Rouget and have since been known as “Rouget’s cells,” and later renamed as “pericytes” in 1923 (Zimmermann, 1923). Located along the basement membrane, pericytes interact with endothelial cells to regulate capillary diameter (Gerhardt and Betsholtz, 2003). Endothelial cells recruit pericytes through PDGF-B/PDGFR-β paracrine signaling during the sprouting of capillaries (Hellstrom et al., 1999; Lindblom et al., 2003).
In adults, SMC in arteries and veins help vessels resist the radial force of the blood flow and have a specialized function of contraction that allows for adjustments in vascular tone to regulate vessel diameter, blood pressure, and blood distribution (Owens et al., 2004). Loss or phenotypic change of SMC is implicated in the pathogenesis of human disease, such as aneurysm formation, atherosclerosis, systemic hypertension, and cancer (Slavin and Gonzalez-Vitale, 1976; Owens et al., 2004).
Of unexpected interest, expression of ephrinB2 defines genetic differences between arteries and veins not only in endothelial cells, but also in mural cells, both during later stages of embryogenesis as well as in adults (Gale et al., 2001; Shin et al., 2001), further supporting the role for ephrinB2 during formation of the arterial medial layer. These observations also suggest distinct mechanisms regulating the timing of onset of ephrinB2 expression in endothelial cells and in mural cells of mesenchymal origin. Notch signaling is also critically involved in SMC development, in addition to its role in arterial differentiation of endothelial cells (Simons and Eichmann, 2015).
Pericytes are increasingly recognized as potential targets for pro- and anti-angiogenic therapies. However, pericyte investigations have been hindered, due to a lack of a standardized and efficient method for isolating these cells. Maier et al. (2010) reported a new method for culturing pericytes from human placentas, which may facilitate future studies.
Membrane Signaling in Human Vascular Physiology and Pathophysiology
VEIN GRAFT ADAPTATION
Vein grafts remain the gold standard conduit used by surgeons as a bypass graft to treat severe arterial occlusive disease. Exposure of the vein graft to arterial pressure, flow, and oxygen tension results in venous adaptive remodeling, namely venous wall thickening and luminal dilation. The remodeling process is a complex balance of cell proliferation and migration, programmed cell death, and changes to the extracellular matrix. These events are guided by well-coordinated signaling pathways activated, at least in part, by exposure of the venous endothelium to increased shear stress exerted by arterial flow (Mitra et al., 2006).
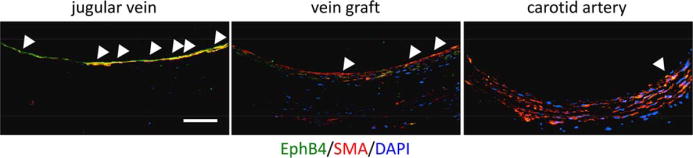
The Eph RTK family is a critical signaling pathway involved in vein graft remodeling. Human studies have shown that expression of the venous determinant, EphB4, is reduced in vein grafts compared with native veins (Kudo et al., 2007) (Fig. 4). Ex vivo study of human saphenous veins confirmed that increased shear stress results in loss of EphB4 expression (Berard et al., 2013; Model et al., 2014). Animal models of vein graft adaptation have also been developed and similarly show reduced EphB4 expression in vein grafts (Mitra et al., 2006; Kudo et al., 2007; Muto et al., 2011).
FIGURE 4.
Representative photomicrographs showing immunoreactivity of EphB4 (green) and smooth muscle actin (SMA) (red), 3 weeks after vein graft placement in aged rats; counterstained with DAPI (blue). White arrowheads indicate positive signals for EphB4. Scale bar 50 μm.
Vein graft EphB4 expression and activity has been manipulated in rodent models to show that EphB4 is an active mediator of vein graft remodeling. An siRNA approach suggested that reduced EphB4 expression was associated with increased vein graft wall thickness, suggesting that EphB4 is an inhibitor of wall thickening (Kudo et al., 2007). Direct stimulation of EphB4 receptors using the specific ligand ephrinB2/Fc resulted in retention of EphB4 expression and thinning of the vein graft wall to more closely approximate an expected venous phenotype (Muto et al., 2011). Similar findings were also shown by overexpressing EphB4 receptors using a viral vector; conversely, reduced EphB4 signaling in EphB4 heterozygous mice was associated with thickening of the vein graft wall (Muto et al., 2011).
The mouse vein graft model also provides evidence for downstream signaling via Cav1 as essential for EphB4-mediated vein graft adaptation (Muto et al., 2011). Cav1 decreases in vein grafts relative to native veins, analogous to the reduced expression of EphB4. Increased expression or activity of EphB4 results in increased Cav1 phosphorylation, whereas decreased EphB4 results in decreased Cav1 phosphorylation. Vein grafts derived from Cav1 knockout mice have reduced wall thickness and are unresponsive to EphB4 specific stimulation, suggesting Cav1 is an essential component of EphB4 signaling during vein graft remodeling (Muto et al., 2011).
NO production is also a downstream mediator of EphB4-dependent vein graft remodeling (Muto et al., 2011; Wang et al., 2015). In vein grafts with reduced EphB4 activity, there is reduced eNOS phosphorylation (Jadlowiec et al., 2013). NO is a known regulator of vein graft wall thickness (Kibbe et al., 2001) and animal models lacking eNOS show inhibited remodeling (Wang et al., 2015).
Growth factors upstream of the Eph-ephrin pathway also influence vein graft adaptation. Among them, VEGF is of particular importance (Hayashi et al., 2005). Mouse models of vein graft adaptation show transiently increased VEGF-A expression and then subsequent down-regulation (Kudo et al., 2007). The consequence of transiently increased vein graft VEGF-A remains unclear as increased VEGF-A has been associated with increased proliferation and intimal hyperplasia in some studies (Kudo et al., 2007), yet has also been shown to be protective by inhibiting vein graft intimal hyperplasia in a rabbit model (Luo et al., 1998).
Whereas venous identity, as identified by EphB4 expression, is lost during vein graft adaptation, arterial identity is not gained. Both human and rat patent vein grafts do not acquire the arterial markers ephrinB2, Dll4, or Notch4 (Kudo et al., 2007), although a recent study reported that Dll4 in macrophages promotes the development of vein graft lesions (Koga et al., 2015). These results suggest that while the vein graft does thicken and remodel in the arterial environment, the vein does not transform into an arterial vessel; the vein graft has neither “arterial” nor “venous” identity.
The mitogen activated protein kinase (MAPK) family promotes cell proliferation, differentiation, and motility, and MAPK signaling is up-regulated during vein graft remodeling. Multiple studies have demonstrated that inhibition of MAPK in animal vein graft models results in reduced neointimal hyperplasia (Zhao et al., 2014; Evans et al., 2015). The phosphatidylinositol 3-kinase-Akt (PI3K-Akt) pathway also modulates vein graft remodeling; PI3k-Akt activation promotes cell survival and protein synthesis by activation of downstream signals, including the mammalian target of rapamycin (mTOR) protein (Mitra et al., 2006).
The functional consequence of these activated signaling pathways is likely to result in SMC activation (Muto et al., 2007); consequently, there is increased SMC deposition and migration from the vein graft media to the intima, although the potential sources of these SMC are controversial. Proposed working models other than in situ differentiation include migration and transdifferentiation from adventitial fibroblasts, monocytes/macrophages, as well as circulating blood-borne stem cells (Welt and Rogers, 2002; Muto et al., 2007). Perivascular fibroblasts may also be converted into myofibroblasts, SMC-like cells that have migratory and synthetic capacity (Shi et al., 1996, 1997). These activated SMC are associated with increased extracellular matrix deposition and contribute to neointimal thickening (Westerband et al., 2001; Siow et al., 2003; Mitra et al., 2006).
After the vein is surgically implanted into the arterial environment, the factors that regulate and distinguish favorable adaptive remodeling from that of pathologic remodeling remain poorly defined (Lu et al., 2014; Owens et al., 2015). Outward remodeling and wall thickening are essential for vein graft maturation and function, yet excessive thickening leads to neointimal hyperplasia and remains the leading cause of vein graft failure (Collins et al., 2012). Pathologic neointimal hyperplasia is thought to be induced by endothelial injury as a result of surgical manipulation, ischemia, and increased shear stress from arterial flow. Early after graft implantation, platelets adhere to areas of endothelial injury and release inflammatory cytokines, including PDGF, TGF-β, cytokines such as IL-1, IL-6, and IL-8, and thrombin (Ishiwata et al., 1997; Muto et al., 2010; Collins et al., 2012; Lu et al., 2014). Leukocytes are then recruited and activated, further promoting an inflammatory response (Owens et al., 2015). Ultimately, SMC become more motile and are recruited to areas of endothelial injury. There is a resultant increase in cellular proliferation and deposition of extracellular matrix at these sites. These events are attenuated by reestablishment of an intact endothelium highlighting the significance of endothelial injury as a trigger for pathologic remodeling (Mitra et al., 2006).
Placenta, Bone, and Cancer
EphrinB2 and EphB4 are also found in tissues such as placenta and bone, as well as in tumors (Table 1).
TABLE 1.
Effects of EphB4 and ephrinB2 in Cancer, Bone, and Placental Physiology
| Source (Cell line) | Findings | References |
|---|---|---|
| Cancers | ||
|
| ||
| Breast Cancer (MCF10-B4) | EphB4 overexpression enhances migration, invasion, and colony formation; ephrinB2 reverses these effects via a forward signaling pathway | Rutkowski et al., 2012 |
|
| ||
| Prostate Cancer | ||
| (PC3, PC3M, DU145, ALVA31, LAPC-4, LNCaP, CWR22R) | EphB4 expression is high in PC3 and higher in its metastatic form, PC3M | Xia et al., 2005, |
| (22Rv1) | EphB4 overexpression enhances migration, invasion, and colony formation; ephrinB2 reverses these effects via a forward signaling pathway | Rutkowski et al., 2012 |
|
| ||
| HNSCC (SCC-15) | Inhibition of EphB4 in tumor cells leads to reduced cell number, apoptosis and activation of the death receptor-caspase pathway | Masood et al., 2006 |
|
| ||
| Melanoma (K1735, SW1, M2, P, C19, C23) | High EphB4 expression enhances metastatic and migratory potential | Yang et al., 2006 |
|
| ||
| Kaposi’s Sarcoma | EphrinB2 is necessary for survival of KS cells | Masood et al., 2005 |
|
| ||
| Angiosarcoma | Increased EphB4 expression | Dill et al., 2012 |
|
| ||
| Skeletal System | ||
|
| ||
| Osx=LacZ+ precursor cells, Col1=LacZ+ mature osteoblasts | Osx=LacZ+ cells closely colocalized with cartilage-invading vessels | Maes et al., 2010 |
|
| ||
| Placenta | ||
|
| ||
| (HIPEC-65, BeWo) | Expression of EphB4 and ephrinB2 is induced by hypoxia (HIPEC-65 and BeWo); induction of ephrinB2 expression is independent of HIF-1alpha (BeWo) | Chennakesava et al., 2006 |
|
| ||
| Severe pre-eclamptic human placentas | Increased miR-17, miR-20a, and miR-20b expression | Wang et al., 2012 |
| (HUVEC, BeWo) | Inhibition of miR-20b expression increased IF1A, MMP2, ephrinB2 and EphB4 expression | |
THE PLACENTA
Successful development and maintenance of the uteroplacental vascular network is vital for the developing embryo and is dependent on the interplay between EphB4 and ephrinB2. In the mouse uterus, EphB4 and ephrinB2 are expressed dynamically in the spiral arteries, uterine natural killer cells, and trophoblasts during gestation days 6.5 to 12.5, suggesting their roles in the modification of spiral arteries and in the mechanisms of pathological pregnancy, due to abnormal spiral artery development (Dong et al., 2016). The placenta is structurally composed of cytotrophoblasts (CTB) and syncytiotrophoblasts (STB) (Mi et al., 2000). STB form the border of the villi in contact with the maternal decidua and facilitate the nutrient and gas exchange between the maternal and fetal environments. CTB, on the other hand, provide a source of proliferating and migratory stem cells that ultimately establish the uteroplacental circulation. In the human placenta, Makikallio et al. (2005) showed that the embryonic blood circulation is present at 7 weeks of development with a drastic augmentation in flow rate, without change of the vascular impedance, after 10 weeks of development. These changes are driven by angiogenesis, vasculogenesis, and their respective mediators.
In exploring the instrumental role of Ephs and Ephrins in the specification of cell fate and migration in the human placenta, Chennakesava, et al. (2006) found that EphB4-ephrinB2 interaction is dependent on gestational age. EphB4 and ephrinB2 are highly expressed in the first trimester placenta, but are diminished at term. At 7 weeks of gestation, EphB4 was preferentially detected in the syncytial cell layer of the villi; the underlying CTB layer was only weakly positive for EphB4. Inside the villi, EphB4 was detected in single mesenchymal cells. Endothelial cells of newly formed capillaries, however, lacked EphB4 receptor expression. Outside the villi, EphB4 expression was highest in the CTB giant cells. Conversely, at 7 weeks, ephrinB2 expression was equally strong in the CTB and STB cell layers of the villi. No difference in distribution was noted in expression between cytoplasm and nuclei of CTB. Expression of both EphB4 and ephrinB2 stabilized by 12 weeks and strongly diminished by term in the human placenta.
The expression pattern of EphB4 and ephrinB2 in the placenta also appears to be associated with some placental diseases. Preeclampsia affects 5 to 8% of all human pregnancies, and is characterized by new onset hypertension and proteinuria (Redman and Sargent, 2005; Kanasaki and Kalluri, 2009). In preeclampsia, fewer trophoblasts invade the uterus, and those that do have shallow penetrance (Goldman-Wohl and Yagel, 2002). Wang et al. (2012) extracted mRNA from villous placental tissues of healthy term and severely pre-eclamptic pregnancies to show that miRNA-17, −20a, and −20b were significantly increased in placentas from pre-eclamptic pregnancies. miR-20b was found to directly target EphB4 and ephrinB2, and was expressed primarily in the syncytium and some of the villous STB in term placentas, but not in the capillary endothelial cells. Interestingly, placental ephrinB2 mRNA was significantly down-regulated in pre-eclampsia compared with normotensive pregnancies.
These studies suggest that the expression of ephrinB2 and EphB4 is controlled, temporally varied, and preferentially expressed by cell type in the establishment of the uteroplacental circulation; their deregulation compromises healthy fetal development and maternal health.
THE SKELETAL SYSTEM
Eph- and Ephrin-mediated signaling is thought to affect progression of several diseases, such as osteoarthritis (Kwan Tat et al., 2008, 2009), rheumatoid arthritis (Kitamura et al., 2008), multiple myeloma (Pennisi et al., 2009), and osteosarcoma (Varelias et al., 2002; Fritsche-Guenther et al., 2010). In bone, Eph-receptor and ephrin-ligand interactions mediate osteoblast and osteoclast proliferation, migration, attachment, spread, and differentiation from precursor cells. In differentiating osteoclasts, mRNAs of ephrinB1 and ephrinB2 were detected; calvarial osteoblasts constitutively expressed several ephrinB ligands, EphB receptors, and EphA4 (Zhao et al., 2006); their interactions function during somitogenesis as well as craniofacial and limb development (Matsuo and Otaki, 2012). Interactions between EphB4 and ephrinB2 also mediate stromal-hematopoietic cell crosstalk during bone remodeling, fracture repair, and arthritis (Zhao et al., 2006; Maes et al., 2010; Valverde-Franco et al., 2012; Nguyen et al., 2015).
During development, mesenchymal cells condense and differentiate into chondrocytes to form avascular cartilage models of future bones. Chondrocytes within cartilage then differentiate into hypertrophic chondrocytes that produce VEGF to stimulate angiogenesis (Matsuo and Otaki, 2012). Osteoblasts appear in the surrounding perichondrium and generate the provisional bone cortex. Vascular invasion of the cartilaginous template ensues and prompts the establishment of the primary ossification center inside the bone. Endothelial cells and osteoclasts accumulate in the perichondrium and invade and erode the cartilage, while osteoblasts and marrow cells populate the highly vascularized area (Karsenty and Wagner, 2002; Kronenberg, 2007). Maes et al. (2010) showed that the entry of EphB4-positive, ephrinB2-positive osteoblast precursors into developing bones is intimately related to their invasion by blood vessels. This finding suggests that there may be coupled movement involving mechanisms that are specific to early cells in the osteoblast lineage and lost upon advanced osteoblastic differentiation.
Osteoblast dual expression of EphB4 and ephrinB2 is likely to be one factor that guides bone development and healing, and aberrant expression or signaling may be a factor in various bone pathologies.
CANCERS
Sustained angiogenesis is one of the many hallmarks of cancer (Hanahan, 2000), and altered patterns of EphB4 and ephrinB2 expression may favor tumor growth and neovascularization. Kaposi sarcoma (KS) is one such example. KS is a multifocal angioproliferative disease of endothelial cell origin (Gill et al., 1994). Masood et al. (2005) showed that KS tissue and cell lines are of arterial phenotype, with abundant expression of ephrinB2, but with little or no EphB4. Infection of venous endothelial cells with HHV-8 or VEGF resulted in a phenotype switch from EphB4 to ephrinB2; inhibition of ephrinB2 using the extracellular domain of EphB4 fused with human serum albumin (sEphB4-HSA) inhibited migration, and invasion of KS cells in vitro (Scehnet et al., 2009).
However, increased EphB4 expression is also associated with some cancers. For example, in head and neck squamous cell carcinomas (HNSCC), six of seven HNSCC cell lines studied (SCC-4, −9, −12, −13, −15, −25, −71) had functional EphB4, regulated by EGFR signaling via Akt (Masood et al., 2006). Xia et al. (2005) found EphB4 expression to be highest in the metastatic form of the prostate cancer cell line 3 (PC3M), and showed EphB4 expression localized to the neoplastic epithelium, while it remained absent in normal glands. Further, gene expression analyses revealed EphB4 was expressed in 64 of 72 (89%) cases.
In a later study looking at both prostate and breast cancer cell lines, Rutkowski et al. (2012) showed that EphB4 overexpressing cells had enhanced anchorage-independent growth, migration, and invasion, characteristics of an aggressive phenotype. Importantly, these effects were reversed in the presence of ephrinB2 that led to a reduction in EphB4 protein levels, demonstrating that ligand-dependent signaling is tumor suppressive.
Evaluating the effects of EphB4’s extracellular domain on the ephrinB2 ligand in tumor cell lines, Noren et al. (2004) showed that EphB4 attracts endothelial cells in vitro and stimulates endothelial cell invasion, survival, and proliferation, all crucial functions for angiogenesis. These data support a model in which EphB4 promotes tumor growth by stimulating angiogenesis via ephrinB2.
Similarly, in highly malignant melanoma cells, Yang et al. (2006) showed that these cells express very high levels of EphB4. The receptor is phosphorylated on tyrosine and subsequently associates with Src; increasing the level of activated EphB4 by treating slowly migrating melanoma cells with ephrinB2/Fc significantly enhanced their migratory ability.
VASCULAR TUMORS
Hemangiomas are benign tumors and the most common tumor of infancy, affecting about 10% of all children (Waner et al., 2003). The tumors proliferate rapidly during the first year of life and then gradually involute. Calicchio et al. (2009) showed that the proliferating phase of hemangiomas is characterized by immature endothelial cells and adjacent pericyte-like cells. Involution, on the other hand, is characterized by endothelial apoptosis, dilation of vascular lumens, flattening of endothelial cells, loss of mitotic activity, thickening of basement membranes, and dropout of capillaries. In proliferating hemangiomas, there was increased transcript expression of Tie2, Jagged1, and Notch4; however, there were increased EphB3, but not EphB4, receptors (Calicchio et al., 2009).
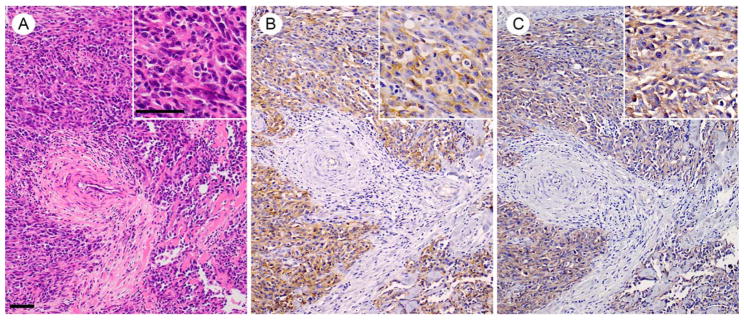
Angiosarcomas are highly malignant vascular tumors of endothelial origin with a mortality rate of 79 to 83%, due to their rapid growth and metastasis (Mark et al., 1996; Lahat et al., 2010); these tumors are currently thought to arise from the transformation of endothelial cells or from circulating stem cells (Young et al., 2010). Immunohistochemical analysis of human skin angiosarcoma samples showed positivity of membranous EphB4 in 42% (10 of 24) of cases (Fig. 5), suggesting a role for EphB4 in the pathogenesis and behavior of angiosarcoma. Signaling upstream of EphB4 is implicated as well; analyses of Notch1 knockout mice showed increased EphB4 expression in liver sinusoidal endothelium, with increased angiogenesis, proliferation, and portal hypertension that resulted in sinusoidal capillarization and angiosarcomas (Dill et al., 2012).
FIGURE 5.
Photomicrographs showing representative histology of human skin angiosarcoma. (A) Hematoxylin and eosin (H&E) staining; (B) CD31 immunolocalization using a mouse monoclonal antibody (catalog no. M0823, Dako); (C) EphB4 immunolocalization using a rabbit monoclonal antibody (catalog no. #14960, Cell Signaling Technology). Assessment of EphB4 immunoreactivity in human skin angiosarcoma showed 42% of cases were positive; n = 24. Scale bars 50 μm.
Hemangiomas and angiosarcomas are two vascular pathologies of endothelial origin affecting different populations and with different outcomes. Though neither is fully understood, their molecular basis revolves around the signaling pathways of vascular identity. Unchecked expression of EphB4 or ephrinB2 in various cancers frequently identifies an aggressive phenotype with important consequences for patients and potentially presenting a target for therapy.
Conclusions and Future Perspectives
Over the past 20 years, there has been significant progress in defining the mechanisms that underlie determination of embryonic vessel identity, with the establishment of Eph-ephrin interactions as critical for vascular development and biology. Several of the signaling pathways that guide embryonic development are also active in adult vessels, and continue to show plasticity and pathology in adults. These findings have been clinically translated with successful inhibition of tumor vessels (Boyd et al., 2014). Continued clinical success for strategies to alter membrane signaling for management of disease will rely on furthering our understanding of the molecular basis for these processes.
Acknowledgments
Dr. Taisuke Mori from the Department of Pathology and Clinical Laboratories, National Cancer Center Hospital (Tokyo), collected human angiosarcoma specimens for tissue microarray.
Supported in part by the National Institutes of Health (R01-HL095498 and R56-HL095498 [to A.D.]); the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program (Merit Review Award I01-BX002336 [to A.D.]); a Sarnoff Cardiovascular Foundation Fellowship (to J.M.S.); Japan Society for the Promotion of Science (JSPS) KAKENHI (No. 15H06879 [to M.T.]); Takeda Science Foundation (No. 2015040635 [to M.T.]); as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
References
- Aboulaich N, Vainonen JP, Stralfors P, Vener AV. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J. 2004;383:237–248. doi: 10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH. Molecular control of arterial-venous blood vessel identity. J Anat. 2003;202:105–112. doi: 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Diella F, Hennig S, et al. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres AC, Reid HH, Zurcher G, et al. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994;9:1461–1467. [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Aspalter IM, Gordon E, Dubrac A, et al. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun. 2015;6:7264. doi: 10.1038/ncomms8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Schlessinger J. Asymmetric tyrosine kinase arrangements in activation or autophosphorylation of receptor tyrosine kinases. Mol Cells. 2010;29:443–448. doi: 10.1007/s10059-010-0080-5. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumori-genesis. Cold Spring Harb Perspect Biol. 2012;4:a008227. doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Zeigler FC, Gu Q, et al. Molecular cloning of a ligand for the EPH-related receptor protein-tyrosine kinase Htk. Proc Natl Acad Sci USA. 1995;92:1866–1870. doi: 10.1073/pnas.92.6.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard X, Deglise S, Alonso F, et al. Role of hemodynamic forces in the ex vivo arterialization of human saphenous veins. J Vasc Surg. 2013;57:1371–1382. doi: 10.1016/j.jvs.2012.09.041. [DOI] [PubMed] [Google Scholar]
- Bergemann AD, Cheng HJ, Brambilla R, et al. ELF-2, a new member of the Eph ligand family, is segmentally expressed in mouse embryos in the region of the hindbrain and newly forming somites. Mol Cell Biol. 1995;15:4921–4929. doi: 10.1128/mcb.15.9.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez P, Sharma A, Bauer PM, et al. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest. 2011;121:3747–3755. doi: 10.1172/JCI44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13:39–62. doi: 10.1038/nrd4175. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calicchio ML, Collins T, Kozakewich HP. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol. 2009;174:1638–1648. doi: 10.2353/ajpath.2009.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Chen Z, Bakhshi FR, Shajahan AN, et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23:1388–1398. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennakesava C, Di Santo S, Ziemiecki A, et al. Differential expression of the receptor tyrosine kinase EphB4 and its ligand Ephrin-B2 during human placental development. Placenta. 2006;27:959–967. doi: 10.1016/j.placenta.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Collins MJ, Li X, Lv W, et al. Therapeutic strategies to combat neointimal hyperplasia in vascular grafts. Expert Rev Cardiovasc Ther. 2012;10:635–647. doi: 10.1586/erc.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley BC, Nevado J, Mellad J, et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra234–227ra234. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, et al. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Cui X, Lu YW, Lee V, et al. Venous endothelial marker COUP-TFII regulates the distinct pathologic potentials of adult arteries and veins. Sci Rep. 2015;5:16193. doi: 10.1038/srep16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Shergill U, Thakur L, et al. Ephrin B2/EphB4 pathway in hepatic stellate cells stimulates Erk-dependent VEGF production and sinusoidal endothelial cell recruitment. Am J Physiol Gastrointest Liver Physiol. 2010;298:G908–G915. doi: 10.1152/ajpgi.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Diehl S, Bruno R, Wilkinson GA, et al. Altered expression patterns of EphrinB2 and EphB2 in human umbilical vessels and congenital venous malformations. Pediatr Res. 2005;57:537–544. doi: 10.1203/01.PDR.0000155761.70710.C4. [DOI] [PubMed] [Google Scholar]
- Dill MT, Rothweller S, Djonov V, et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology. 2012;142:967–977. e2. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- Dong H, Yu C, Mu J, et al. Role of EFNB2/EPHB4 signaling in spiral artery development during pregnancy: An appraisal. Mol Reprod Dev. 2016;83:12–18. doi: 10.1002/mrd.22593. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Dupree P, Parton RG, Raposo G, et al. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Del Pozo MA. Caveolae - mechanosensitive membrane invaginations linked to actin filaments. J Cell Sci. 2015;128:2747–2758. doi: 10.1242/jcs.153940. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Evans BC, Hocking KM, Osgood MJ, et al. MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia. Sci Transl Med. 2015;7:291ra295–291ra295. doi: 10.1126/scitranslmed.aaa4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancher TT, Muto A, Fitzgerald TN, et al. Control of blood vessel identity: from embryo to adult. Ann Vasc Dis. 2008;1:28–34. doi: 10.3400/avd.AVDrev07011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, et al. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Fielding PE, Chau P, Liu D, et al. Mechanism of platelet-derived growth factor-dependent caveolin-1 phosphorylation: relationship to sterol binding and the role of serine-80. Biochemistry. 2004;43:2578–2586. doi: 10.1021/bi035442c. [DOI] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Fritsche-Guenther R, Noske A, Ungethum U, et al. De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology. 2010;57:836–850. doi: 10.1111/j.1365-2559.2010.03713.x. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- García-Cardeña G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Oh P, Liu J, et al. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- Gill PS, Hamilton A, Naldu Y. Epidemic (AIDS-related) Kaposi’s sarcoma: epidemiology pathogenesis and treatment. AIDS Updates. 1994;7:1–11. [Google Scholar]
- Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–20166. [PubMed] [Google Scholar]
- Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1992;89:10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol. 2002;187:233–238. doi: 10.1016/s0303-7207(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Gratton J-P, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Prince V, Lumsden A. Selective dispersal of avian rhombomere cells in orthotopic and heterotopic grafts. Development. 1993;118:527–538. doi: 10.1242/dev.118.2.527. [DOI] [PubMed] [Google Scholar]
- Hanahan DW, RA The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Howard G, Nichols BJ. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J Cell Sci. 2011;124:2777–2785. doi: 10.1242/jcs.084319. [DOI] [PubMed] [Google Scholar]
- Hayashi S-i, Asahara T, Masuda H, et al. Functional Ephrin-B2 expression for promotive interaction between arterial and venous vessels in postnatal neovascularization. Circulation. 2005;111:2210–2218. doi: 10.1161/01.CIR.0000163566.07427.73. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hellström M, Phng L-K, Gerhardt H. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adh Migr. 2007;1:133–136. doi: 10.4161/cam.1.3.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Bastiani M, Luetterforst R, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, et al. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Hu S, Wilson KD, Ghosh Z, et al. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells. 2013;31:259–268. doi: 10.1002/stem.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S, Tukada T, Nakanishi S, et al. Postangioplasty restenosis: platelet activation and the coagulation-fibrinolysis system as possible factors in the pathogenesis of restenosis. Am Heart J. 1997;133:387–392. doi: 10.1016/s0002-8703(97)70178-9. [DOI] [PubMed] [Google Scholar]
- Jadlowiec CC, Feigel A, Yang C, et al. Reduced adult endothelial cell EphB4 function promotes venous remodeling. Am J Physiol Cell Physiol. 2013;304:C627–C635. doi: 10.1152/ajpcell.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Tao M, Omalley KA, et al. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am J Physiol Heart Circ Physiol. 2009;297:H1200–H1207. doi: 10.1152/ajpheart.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B, Bastiani M, Strugnell SS, et al. Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation. J Cell Biol. 2012;199:425–435. doi: 10.1083/jcb.201207089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Jurisic G, Detmar M. Lymphatic endothelium in health and disease. Cell Tissue Res. 2009;335:97–108. doi: 10.1007/s00441-008-0644-2. [DOI] [PubMed] [Google Scholar]
- Kalo MS, Pasquale EB. Signal transfer by Eph receptors. Cell Tissue Res. 1999;298:1–9. [PubMed] [Google Scholar]
- Kanasaki K, Kalluri R. The biology of preeclampsia. Kidney Int. 2009;76:831–837. doi: 10.1038/ki.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TJ, Law C, Kania A. Eph and ephrin signaling: lessons learned from spinal motor neurons. Semin Cell Dev Biol. 2012;23:83–91. doi: 10.1016/j.semcdb.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kibbe MR, Tzeng E, Gleixner SL, et al. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim S, Koh H, et al. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- Kim YN, Wiepz GJ, Guadarrama AG, Bertics PJ. Epidermal growth factor-stimulated tyrosine phosphorylation of caveolin-1. Enhanced caveolin-1 tyrosine phosphorylation following aberrant epidermal growth factor receptor status. J Biol Chem. 2000;275:7481–7491. doi: 10.1074/jbc.275.11.7481. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kabuyama Y, Kamataki A, et al. Enhancement of lymphocyte migration and cytokine production by ephrinB1 system in rheumatoid arthritis. Am J Physiol Cell Physiol. 2008;294:C189–196. doi: 10.1152/ajpcell.00314.2007. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signalling during development. Development. 2012;139:4105–4109. doi: 10.1242/dev.074997. [DOI] [PubMed] [Google Scholar]
- Koga J, Nakano T, Dahlman JE, et al. Macrophage notch ligand delta-like 4 promotes vein graft lesion development: implications for the treatment of vein graft failure. Arterioscler Thromb Vasc Biol. 2015;35:2343–2353. doi: 10.1161/ATVBAHA.115.305516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Muto A, Kudo FA, et al. Age-related Notch-4 quiescence is associated with altered wall remodeling during vein graft adaptation. J Surg Res. 2011;171:e149–e160. doi: 10.1016/j.jss.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. The role of the perichondrium in fetal bone development. Ann NY Acad Sci. 2007;1116:59–64. doi: 10.1196/annals.1402.059. [DOI] [PubMed] [Google Scholar]
- Kruse SW, Suino-Powell K, Zhou XE, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo FA, Muto A, Maloney SP, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27:1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- Kwan Tat S, Pelletier JP, Amiable N, et al. Activation of the receptor EphB4 by its specific ligand ephrin B2 in human osteo-arthritic subchondral bone osteoblasts. Arthritis Rheum. 2008;58:3820–3830. doi: 10.1002/art.24029. [DOI] [PubMed] [Google Scholar]
- Kwan Tat S, Pelletier JP, Amiable N, et al. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Res Ther. 2009;11:R119. doi: 10.1186/ar2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat G, Dhuka AR, Hallevi H, et al. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010;251:1098–1106. doi: 10.1097/SLA.0b013e3181dbb75a. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Partridge EA, Guay G, et al. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol. 2007;179:341–356. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner F, Sohl M, Farnebo F. Functional arterial and venous fate is determined by graded VEGF signaling and notch status during embryonic stem cell differentiation. Arterioscler Thromb Vasc Biol. 2007;27:487–493. doi: 10.1161/01.ATV.0000255990.91805.6d. [DOI] [PubMed] [Google Scholar]
- Larrivee B, Freitas C, Suchting S, et al. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lee H, Volonte D, Galbiati F, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Chen EY, Wong DJ, et al. Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res. 2014;188:162–173. doi: 10.1016/j.jss.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Asahara T, Tsurumi Y, et al. Reduction of vein graft intimal hyperplasia and preservation of endothelium-dependent relaxation by topical vascular endothelial growth factor. J Vasc Surg. 1998;27:167–173. doi: 10.1016/s0741-5214(98)70304-0. [DOI] [PubMed] [Google Scholar]
- Lynch PM, Delano FA, Schmid-Schonbein GW. The primary valves in the initial lymphatics during inflammation. Lymphat Res Biol. 2007;5:3–10. doi: 10.1089/lrb.2007.5102. [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CL, Shepherd BR, Yi T, Pober JS. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation. 2010;17:367–380. doi: 10.1111/j.1549-8719.2010.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makikallio K, Jouppila P, Rasanen J. Human fetal cardiac function during the first trimester of pregnancy. Heart. 2005;91:334–338. doi: 10.1136/hrt.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma: a report of 67 patients and a review of the literature. Cancer. 1996;77:2400–2406. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Masood R, Kumar SR, Sinha UK, et al. EphB4 provides survival advantage to squamous cell carcinoma of the head and neck. Int J Cancer. 2006;119:1236–1248. doi: 10.1002/ijc.21926. [DOI] [PubMed] [Google Scholar]
- Masood R, Xia G, Smith L, et al. EphrinB2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: phenotype switch from venous to arteral endothelium. Blood. 2005;105:1311–1318. doi: 10.1182/blood-2004-03-0933. [DOI] [PubMed] [Google Scholar]
- Mastick CC, Brady MJ, Saltiel AR. Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol. 1995;129:1523–1531. doi: 10.1083/jcb.129.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone remodeling, remodeling and associated diseases. Cell Adh Migr. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- Model LS, Hall MR, Wong DJ, et al. Arterial shear stress reduces Eph-B4 expression in adult human veins. Yale J Biol Med. 2014;87:359–371. [PMC free article] [PubMed] [Google Scholar]
- Moses HL, Serra R. Regulation of differentiation by TGF-beta. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, et al. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- Murtomaki A, Uh MK, Choi YK, et al. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development. 2013;140:2365–2376. doi: 10.1242/dev.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Fitzgerald TN, Pimiento JM, et al. Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons. J Vasc Surg. 2007;45(Suppl A):A15–A24. doi: 10.1016/j.jvs.2007.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Model L, Ziegler K, et al. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010;74:1501–1512. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Yi T, Harrison KD, et al. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J Exp Med. 2011;208:561–575. doi: 10.1084/jem.20101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto M, Cheng HJ, Friedman GC, et al. Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- Nassoy P, Lamaze C. Stressing caveolae new role in cell mechanics. Trends Cell Biol. 2012;22:381–389. doi: 10.1016/j.tcb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Arthur A, Panagopoulos R, et al. EphB4 expressing stromal cells exhibit an enhanced capacity for hematopoietic stem cell maintenance. Stem Cells. 2015;33:2838–2849. doi: 10.1002/stem.2069. [DOI] [PubMed] [Google Scholar]
- Noren NK, Lu M, Freeman AL, et al. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci USA. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Orioli D, Klein R. The Eph receptor family: axonal guidance by contact repulsion. Trends Genet. 1997;13:354–359. doi: 10.1016/s0168-9525(97)01220-1. [DOI] [PubMed] [Google Scholar]
- Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. J Vasc Surg. 2015;61:203–216. doi: 10.1016/j.jvs.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pandey A, Duan H, Dixit VM. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J Biol Chem. 1995;270:19201–19204. doi: 10.1074/jbc.270.33.19201. [DOI] [PubMed] [Google Scholar]
- Pandey A, Lazar DF, Saltiel AR, Dixit VM. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J Biol Chem. 1994;269:30154–30157. [PubMed] [Google Scholar]
- Park BK, Zeng X, Glazer RI. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001;61:7647–7653. [PubMed] [Google Scholar]
- Parton RG. Cell biology. Life without caveolae. Science. 2001;293:2404–2405. doi: 10.1126/science.1065677. [DOI] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pennisi A, Ling W, Li X, et al. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009;114:1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH. Regulation of signaling interactions and receptor endocytosis in growing blood vessels. Cell Adh Migr. 2014;8:366–377. doi: 10.4161/19336918.2014.970010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol A, Martin S, Fernandez MA, et al. Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol Biol Cell. 2004;15:99–110. doi: 10.1091/mbc.E03-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Qin J, Wu SP, Creighton CJ, et al. COUP-TFII inhibits TGF-beta-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–240. doi: 10.1038/nature11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillien A, Moore JC, Shin M, et al. Distinct Notch signaling outputs pattern the developing arterial system. Development. 2014;141:1544–1552. doi: 10.1242/dev.099986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288:H936–H945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- Razani B, Lisanti MP. Caveolin-deficient mice: insights into caveolar function human disease. J Clin Invest. 2001;108:1553–1561. doi: 10.1172/JCI14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Rivera M, Muto A, Feigel A, et al. Venous and arterial identity: a role for caveolae? Vascular. 2009;17(Suppl 1):S10–S14. doi: 10.2310/6670.2008.00088. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Morton C, DePaola N, et al. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Heart Circ Physiol. 2003;285:H1720–H1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothuizen TC, Wong C, Quax PH, et al. Arteriovenous access failure: more than just intimal hyperplasia? Nephrol Dial Transplant. 2013;28:1085–1092. doi: 10.1093/ndt/gft068. [DOI] [PubMed] [Google Scholar]
- Rutkowski R, Mertens-Walker I, Lisle JE, et al. Evidence for a dual function of EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand. Int J Cancer. 2012;131:E614–E624. doi: 10.1002/ijc.27392. [DOI] [PubMed] [Google Scholar]
- Scallan JP, Wolpers JH, Davis MJ. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol. 2013;591:443–459. doi: 10.1113/jphysiol.2012.237909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scehnet JS, Ley EJ, Krasnoperov V, et al. The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma and implications of EphrinB2 blockade. Blood. 2009;113:254–263. doi: 10.1182/blood-2008-02-140020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lewis RY, Volonte D, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol. 1999;277:H1167–H1177. doi: 10.1152/ajpheart.1999.277.3.H1167. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Garcia-Cardena G, Liu J, et al. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shi Y, O’Brien JE, Fard A, et al. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- Shi Y, O’Brien JE, Jr, Mannion JD, et al. Remodeling of auto-logous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95:2684–2693. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Affolter M, Belting HG. The tip cell concept 10 years after: new players tune in for a common theme. Exp Cell Res. 2013;319:1255–1263. doi: 10.1016/j.yexcr.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Simons M, Eichmann A. Molecular controls of arterial morphogenesis. Circ Res. 2015;116:1712–1724. doi: 10.1161/CIRCRESAHA.116.302953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Koster D, Ruez R, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59:212–221. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- Slavin RE, Gonzalez-Vitale JC. Segmental mediolytic arteritis: a clinical pathologic study. Lab Invest. 1976;35:23–29. [PubMed] [Google Scholar]
- Smart EJ, Graf GA, McNiven MA, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- Stapor PC, Sweat RS, Dashti DC, et al. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51:163–174. doi: 10.1159/000362276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Cerretti DP, Daniel TO. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–23593. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Meininger CJ, Forough R, et al. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2002;277:43830–43835. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Nakayama M, Goto TM, et al. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem Biophys Res Commun. 2007;361:462–467. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- Takamoto N, You LR, Moses K, et al. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development. 2005;132:2179–2189. doi: 10.1242/dev.01808. [DOI] [PubMed] [Google Scholar]
- Takayanagi T, Crawford KJ, Kobayashi T, et al. Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin Sci. 2014;126:785–794. doi: 10.1042/CS20130660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Valverde-Franco G, Pelletier JP, Fahmi H, et al. In vivo bone-specific EphB4 overexpression in mice protects both sub-chondral bone and cartilage during osteoarthritis. Arthritis Rheum. 2012;64:3614–3625. doi: 10.1002/art.34638. [DOI] [PubMed] [Google Scholar]
- Varelias A, Koblar SA, Cowled PA, et al. Human osteosarcoma expresses specific ephrin profiles: implications for tumori-genicity and prognosis. Cancer. 2002;95:862–869. doi: 10.1002/cncr.10749. [DOI] [PubMed] [Google Scholar]
- Vihanto MM, Vindis C, Djonov V, et al. Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J Cell Sci. 2006;119:2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- Walser PJ, Ariotti N, Howes M, et al. Constitutive formation of caveolae in a bacterium. Cell. 2012;150:752–763. doi: 10.1016/j.cell.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Waner M, North PE, Scherer KA, et al. The nonrandom distribution of facial hemangiomas. Arch Dermatol. 2003;139:869–875. doi: 10.1001/archderm.139.7.869. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang M, Collins M, Foster T, et al. Eph-B4 mediates vein graft adaptation by regulation of eNOS. J Vasc Surg. 2015;62:792–792. doi: 10.1016/j.jvs.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Feng L, Zhang H, et al. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, −20a, and −20b) that target ephrin-B2 and EphB4 in human placenta. J Clin Endocrinol Metab. 2012;97:E1051–E1059. doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- Westerband A, Crouse D, Richter LC, et al. Vein adaptation to arterialization in an experimental model. J Vasc Surg. 2001;33:561–569. doi: 10.1067/mva.2001.112230. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Lu DY, Protack CD, et al. Ephrin type-B receptor 4 activation reduces neointimal hyperplasia in human saphenous vein in vitro. J Vasc Surg. 2014;63:795–804. doi: 10.1016/j.jvs.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Ashton AW, Schubert W, et al. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003;162:2059–2068. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Kumar SR, Masood R, et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Carrasco R, Kinneer K, et al. EphB4 promotes or suppresses Ras/MEK/ERK pathway in a context-dependent manner: implications for EphB4 as a cancer target. Cancer Biol Ther. 2012;13:630–637. doi: 10.4161/cbt.20080. [DOI] [PubMed] [Google Scholar]
- Xu C, Hasan SS, Schmidt I, et al. Arteries are formed by vein-derived endothelial tip cells. Nat Commun. 2014;5:5758. doi: 10.1038/ncomms6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Alldus G, Holder N, Wilkinson DG. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hind-brain. Development. 1995;121:4005–4016. doi: 10.1242/dev.121.12.4005. [DOI] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Xu Z, Yu S, Hsu CH, et al. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc Natl Acad Sci USA. 2008;105:2421–2426. doi: 10.1073/pnas.0707082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Toya Y, Schwencke C, et al. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–26968. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- Yang N, Pasquale EB, Owen LB, Ethell IM. The EphB4 receptor-tyrosine kinase promotes the migration of melanoma cells thorugh Rho-mediated actin cytoskeleton reorganization. J Biol Chem. 2006;281:32574–32586. doi: 10.1074/jbc.M604338200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest. 2014;124:888–897. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L-R, Lin F-J, Lee CT, et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Peng F, Wu D, et al. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zhao Z-W, Abendroth D-K, Zhou Z-C, et al. Anti-inflammatory and anti-proliferative effects of CBS3830 in arterialized vein grafts in rats. Immunopharmacol Immunotoxicol. 2014;36:397–403. doi: 10.3109/08923973.2014.956754. [DOI] [PubMed] [Google Scholar]
- Zisch AH, Kalo MS, Chong LD, Pasquale EB. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene. 1998;16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]
- Zimmermann KW. Der feinere Bau der Blut-kapillaren. Z Anat. 1923;68:29–109. [Google Scholar]