Abstract
Study Objective
To assess the impact of empiric Pseudomonas pharmacotherapy on 30-day mortality in hospitalized patients with community-onset pneumonia stratified according to their risk (low, medium, or high) of drug-resistant pathogens.
Design
Retrospective cohort study.
Data Source
Veterans Health Administration database.
Patients
A total of 50,119 patients who were at least 65 years of age, hospitalized with pneumonia, and received antibiotics within 48 hours of admission between fiscal years 2002 and 2007; patients were stratified into empiric Pseudomonas therapy (31,027 patients) and no Pseudomonas therapy (19,092 patients) groups based on antibiotics received during their first 48 hours of admission.
Measurements and Main Results: Results
A recently developed clinical prediction scoring system that stratifies patients with community-onset pneumonia according to their risk of drug-resistant pathogens was used to identify patients who were likely to benefit from empiric Pseudomonas therapy as well as those in whom antipseudomonal therapy could be spared; patients were classified into low-risk (68%), medium-risk (21%), and high-risk (11%) groups. Of the 50,119 patients, 62% received Pseudomonas therapy. All-cause 30-day mortality was the primary outcome. Empiric Pseudomonas therapy (adjusted odds ratio 0.72, 95% confidence interval 0.62-0.84) was associated with lower 30-day mortality in the high-risk group but not the low- or medium-risk groups.
Conclusion
Application of a risk score for patients with drug-resistant pathogens can identify patients likely to benefit from empiric Pseudomonas therapy. Widespread use of this score could reduce overuse of anti-Pseudomonas antibiotics in low- to medium-risk patients and improve survival in high-risk patients.
Keywords: evidence-based medicine, infectious disease, outcomes, pharmacy practice, pulmonary
Pneumonia continues to be the leading cause of mortality due to infectious disease in the United States, with 53,826 pneumonia-related deaths in 2011.1 The American Thoracic Society (ATS)/Infectious Diseases Society of America's (IDSA) guidelines for community-acquired pneumonia (CAP) described four variants of pneumonia: CAP, hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), and health care–associated pneumonia (HCAP).2 The 2005 ATS/IDSA guidelines for HCAP/HAP/VAP suggested that clinicians manage patients with HCAP patients similarly to patients with HAP and VAP because it was believed at the time that patients with HCAP risk factors were at similar risk of multidrug-resistant (MDR) pathogens as patients with HAP and VAP.3 That approach has now fallen out of favor. The 2016 HAP/VAP guidelines acknowledge that there is growing evidence that many patients with HCAP are not at risk for MDR pathogens.4 These new guidelines suggest that clinicians use validated risk factors for MDR pathogens, rather than solely relying on HCAP risk factors, when deciding which patients should receive therapy directed at MDR pathogens.4 The guideline authors suggest that the concept of HCAP be discussed in future revisions of the CAP guidelines; those guidelines are currently being revised.
Community-onset (CO) pneumonia is a term used to describe pneumonia that is present 48 hours into hospital admission, with the origin from outside the hospital or health care facility; this term includes both CAP and HCAP. The chief cause of CO-pneumonia is Streptococcus pneumoniae; however, Pseudomonas aeruginosa accounts for 1-2% of CAP cases, 2-8% of severe CAP cases, and 2-26% of HCAP cases.5-13 The antibiotics recommended in the CAP guidelines for common CAP pathogens lack activity against Pseudomonas aeruginosa.2 The antibiotics recommended in the HCAP guidelines do possess activity against Pseudomonas aeruginosa, but this broad-spectrum therapy may be unnecessary for many patients with CO-pneumonia.14,15 Using HCAP criteria to identify patients with possible Pseudomonas is an imprecise method and frequently leads to overtreatment of the general HCAP population and possibly undertreatment of patients with pneumonia variants other than HCAP who would benefit from Pseudomonas therapy.14,15 Furthermore, a large number of patients remain on the initial empiric regimen without changes to a definitive therapeutic treatment once the pathogen is known.16 All of these factors indicate the great need for a reliable method to assess Pseudomonas risk on hospital admission in patients with CO-pneumonia.
Recently, Ma et al developed a clinical prediction scoring system that stratified patients with CO-pneumonia according to their risk of drug-resistant pathogens.17 The total score ranges from 0–21.5, and patients were stratified into low-risk (0.0-2.0), medium-risk (2.5-7.0), and high-risk (7.5-21.5) groups. The drug-resistant pathogen prevalence ranged from less than 10% in the low-risk group to nearly 50% in the high-risk group. Additionally, Pseudomonas was the most common drug-resistant pathogen identified in this study. These findings suggest that use of the risk score could help identify those patients at low risk of Pseudomonas infection, for whom antipseudomonal therapy could be spared, as well as those patients at high risk of Pseudomonas infection, for whom empiric antipseudomonal therapy might be beneficial. A similar claim was made for the antibiotics recommended by the HCAP guidelines;3 however, follow-up studies failed to demonstrate a survival benefit when all patients with HCAP received the broad-spectrum therapies recommended by the guidelines.18,19
The new risk score developed by Ma et al17 could help guide empiric Pseudomonas therapy; however, studies are needed to determine if a tailored regimen including antipseudomonal antibiotics will result in better outcomes, and which, if any, of the risk groups benefit from such therapy. Our primary objective was to compare the effect of empiric Pseudomonas therapy on 30-day mortality among pneumonia patients in the three risk groups defined by Ma et al17 (low, medium, and high risk).
Methods
This study used administrative data from the Veterans Health Administration (VHA) database. Descriptions of the methods used to build this database have been previously reported.18,20,21 In brief, we performed a retrospective, population-based cohort study using administrative data from the VHA system between fiscal years 2002 and 2007. These data are obtained from over 150 VHA hospitals and 1,400 VHA outpatient clinics. Data for this study were obtained from the VHA electronic medical record system that includes administrative, clinical, laboratory, and pharmacy data. The Institutional Review Board of the University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development committee approved this study.
Patients were included if they were at least 65 years of age and had either a primary discharge diagnosis of pneumonia/influenza (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 480.0–483.99 or 485–487) or a secondary discharge diagnosis of pneumonia/influenza plus a primary diagnosis of respiratory failure (ICD-9-CM code 518.81) or sepsis (ICD-9-CM code 038.xx) in fiscal years 2002 to 2007. If a patient was admitted more than once during the study period, only the first hospitalization was included. Patients were excluded if they did not receive antimicrobial therapy within the first 48 hours of admission.
Baseline demographics were recorded at the time of admission. Antibiotic use was recorded for the first 48 hours of admission. Table 1 includes a complete list of all antibiotics considered in this study. We searched for the active ingredient (generic name), so this would include all formulations. Table 1 also includes our definitions of guideline-concordant CAP therapy, Pseudomonas therapy, and methicillin-resistant Staphylococcus aureus (MRSA) therapy. Comorbid conditions were determined using ICD-9-CM codes from outpatient and inpatient care in accordance with the Charlson comorbidity scoring system.22,23
Table 1. Definitions of Antibiotic Therapy for Guideline-Concordant Community-Acquired Pneumonia, Pseudomonas Infections, and MRSA Infections.
| Guideline-concordant community-acquired pneumonia therapy | |
|---|---|
|
| |
| Ward patients | ICU patients |
|
| |
| Pseudomonas therapy | |
|
| |
|
| |
| MRSA therapy | |
|
| |
| |
MRSA = methicillin-resistant Staphylococcus aureus; ICU = intensive care unit
β-Lactams include cefotaxime, ceftriaxone, ampicillin-sulbactam, ertapenem, or aztreonam.
Macrolides include azithromycin, clarithromycin, or erythromycin.
Respiratory fluoroquinolones include moxifloxacin, levofloxacin, or gatifloxacin.
Antipseudomonal β-lactams include cefepime, ceftazidime, imipenem-cilastatin, meropenem, piperacillin-tazobactam, ticarcillin-clavulanate, or aztreonam.
Antipseudomonal fluoroquinolones include ciprofloxacin or levofloxacin.
Aminoglycosides include gentamicin, tobramycin, or amikacin.
The risk score variables were defined as follows: respiratory organ failure (14 points), hospitalization in the past 90 days (5 points), invasive mechanical ventilation (2 points), and one or more HCAP risk factors (0.5 point). HCAP risk factors were defined as hospital admission in the previous 90 days, residence in a nursing home in the previous 90 days, receipt of outpatient intravenous antibiotics in the previous 90 days, and hemodialysis.18 These risk score variables were based on the risk score developed by Ma et al17 and were modified for our database. We had to make some changes to the variable definitions from the study by Ma et al17 to fit our data resource. We had to change the “bronchiectasis” variable (14 points) to a respiratory organ failure variable (14 points) because the presence or absence of bronchiectasis was not available in our database. We changed the “severe pneumonia” variable (2 points) to an “invasive mechanical ventilation” variable (2 points) because our database did not contain CURB-65 scores (severity scores for CAP). This likely resulted in a slightly different population of patients who met our modified rule compared with the rule published by Ma et al.17 Patients were stratified into three risk groups based on their risk score: low (0-2.0), medium (2.5-7.0), and high (7.5-21.5).
Patients were stratified into Pseudomonas therapy and no Pseudomonas therapy groups based on the antibiotics received during their first 48 hours of admission. Pseudomonas therapies were defined as the receipt of specific β-lactams, fluoroquinolones, or aminoglycosides with Pseudomonas activity within the first 48 hours of admission (Table 1). Patients were also categorized based on the receipt of guideline-concordant CAP (GC-CAP) therapy and MRSA therapy (Table 1).2
All-cause 30-day mortality was the primary outcome. Previous research has demonstrated that 30-day mortality is more closely associated with pneumonia-related mortality, compared to 60-day or 90-day mortality.24 Mortality was assessed using the VHA vital status file, which has been demonstrated to have 98% exact agreement with the National Death Index, the “gold standard” to determine mortality.25
Statistical Analysis
All statistical analyses were conducted by using JMP 10.0 statistical software (SAS Institute Inc., Cary, NC). The χ2 or Fisher exact test were used to compare categorical variables between study groups. Continuous variables were compared by using the Wilcoxon Rank Sum test (Table 2). For bivariable statistical tests, we defined significance as a two-tailed α ≤ 0.0001 to avoid spurious associations in this large patient cohort.
Table 2. Baseline Characteristics of the Study Patients.
| Characteristic | No Pseudomonas therapy group (n=19,092) | Pseudomonas therapy group (n=31,027) | p Value |
|---|---|---|---|
| Patient age (yrs), median (IQR) | 78 (72-83) | 78 (72-82) | 0.3247 |
| Male, % | 98 | 98 | 0.2048 |
| Race, % | |||
| White | 84 | 82 | <0.0001 |
| Black | 10 | 13 | <0.0001 |
| Other | 6 | 5 | 0.0119 |
| Hispanic ethnicity, % | 5 | 7 | <0.0001 |
| MDR risk score variables,a % | |||
| Respiratory organ failure (14 points) | 10 | 11 | <0.0001 |
| Hospitalization in the past 90 days (5 points) | 19 | 27 | <0.0001 |
| Invasive mechanical ventilation (2 points) | 5 | 7 | <0.0001 |
| Health care–associated pneumonia risk factor (0.5 point) | 32 | 39 | <0.0001 |
| MDR risk score, median (IQR) | 0 (0-5.5) | 0.5 (0-5.5) | <0.0001 |
| Low (0-2.0), % | 74 | 65 | <0.0001 |
| Medium (2.5-7.0), % | 16 | 23 | <0.0001 |
| High (7.5-21.5), % | 10 | 12 | <0.0001 |
| Charlson comorbidity score, median (IQR) | 2 (1-4) | 2 (1-4) | <0.0001 |
| Comorbid conditions, % | |||
| Myocardial infarction | 7 | 7 | 0.7049 |
| Heart failure | 26 | 26 | 0.2341 |
| Chronic obstructive pulmonary disease | 54 | 53 | 0.1707 |
| Liver disease | 1 | 1 | 0.2977 |
| Renal disease | 12 | 13 | 0.2966 |
| Diabetes mellitus | 33 | 33 | 0.4898 |
| Neoplastic disease | 24 | 27 | <0.0001 |
| HIV/AIDS | <1 | <1 | 0.0206 |
| Medication use within 90 days, % | |||
| Cardiovascular medications | 74 | 69 | <0.0001 |
| Antidiabetic medications | 24 | 23 | 0.0023 |
| Inhaled corticosteroids | 25 | 23 | <0.0001 |
| Systemic corticosteroidsb | 23 | 24 | 0.0033 |
| Pulmonary medications | 40 | 39 | 0.0009 |
| Vasopressors, % | 4 | 5 | <0.0001 |
| Invasive mechanical ventilation, % | 5 | 7 | <0.0001 |
| Noninvasive mechanical ventilation, % | 3 | 4 | <0.0001 |
| Hemodialysis, % | 15 | 16 | 0.0076 |
| Organ failure, % | |||
| Any organ failure | 23 | 25 | <0.0001 |
| Respiratory | 10 | 11 | <0.0001 |
| Cardiovascular | 5 | 6 | 0.0327 |
| Neurologic | 2 | 2 | 0.0080 |
| Renal | 14 | 14 | 0.8298 |
| Hematologic | 2 | 3 | <0.0001 |
| Hepatic | <1 | <1 | 0.9712 |
| Antibiotic therapy, % | |||
| Guideline-concordant community-acquired pneumonia therapy | 77 | 81 | <0.0001 |
| MRSA therapy | 5 | 51 | <0.0001 |
Data are median (interquartile range) or percentages of patients.
HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome; MDR = multidrug-resistant; MRSA: methicillin-resistant Staphylococcus aureus.
The total MDR risk score ranged from 0–21.5 points.
Includes oral and/or injectable corticosteroids.
Separate multivariable logistic regression models were constructed to examine if Pseudomonas therapy was associated with 30-day mortality in the overall population and additionally in each of the three risk groups. The dependent variable was 30-day mortality, and the independent variable was Pseudomonas therapy versus no Pseudomonas therapy. Covariates included all characteristics listed in Table 3. Adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were calculated; those 95% CIs that did not cross one were considered to be statistically significant.
Table 3. Multivariable Models to Identify Risk Factors for 30-Day Mortality, Including Pseudomonas Therapy versus No Pseudomonas Therapy, Stratified by Risk Score.
| Variable | All patients (n=50,119) | Low-risk group (risk score 0-2.0) (n=34,166) | Medium-risk group (risk score 2.5-7.0) (n=10,387) | High-risk group (risk score 7.5-21.5 (n=5,566) |
|---|---|---|---|---|
| Pseudomonas therapy vs no Pseudomonas therapy | 1.23 (1.15-1.31) | 1.44 (1.31-1.59) | 1.23 (1.07-1.40) | 0.72 (0.62-0.84) |
| Age (1-yr increments) | 0.91 (0.90-0.92) | 0.90 (0.89-0.91) | 0.92 (0.91-0.94) | 0.91 (0.89-0.93) |
| Racea | 1.00 (0.92-1.09) | 0.94 (0.83-1.07) | 1.03 (0.88-1.21) | 1.00 (0.83-1.19) |
| Hispanic ethnicity | 0.95 (0.85-1.06) | 1.03 (0.88-1.20) | 0.80 (0.65-0.99) | 0.98 (0.77-1.24) |
| Comorbid conditions | ||||
| Myocardial infarction | 1.14 (1.02-1.26) | 1.08 (0.90-1.30) | 0.95 (0.80-1.12) | 1.11 (0.90-1.37) |
| Heart failure | 1.10 (1.03-1.18) | 1.01 (0.92-1.12) | 1.00 (0.89-1.13) | 1.08 (0.94-1.23) |
| COPD | 0.96 (0.90-1.03) | 0.98 (0.89-1.07) | 0.94 (0.83-1.06) | 0.88 (0.77-1.01) |
| Liver disease | 1.45 (1.13-1.87) | 1.45 (0.94-2.15) | 1.17 (0.75-1.77) | 1.78 (1.06-3.10) |
| Renal disease | 0.98 (0.90-1.07) | 0.96 (0.84-1.09) | 0.90 (0.78-1.04) | 0.99 (0.83-1.18) |
| Diabetes mellitus | 1.03 (0.95-1.12) | 1.05 (0.93-1.19) | 1.00 (0.86-1.17) | 1.03 (0.86-1.22) |
| Neoplastic disease | 1.69 (1.59-1.79) | 1.55 (1.42-1.69) | 1.83 (1.64-2.04) | 1.46 (1.29-1.67) |
| Medication use | ||||
| Cardiovascular medications | 0.71 (0.67-0.76) | 0.67 (0.61-0.73) | 0.68 (0.60-0.77) | 0.84 (0.74-0.96) |
| Antidiabetic medications | 0.90 (0.82-1.00) | 0.81 (0.70-0.94) | 1.04 (0.87-1.23) | 0.93 (0.77-1.14) |
| Inhaled corticosteroids | 0.72 (0.67-0.78) | 0.69 (0.61-0.78) | 0.68 (0.58-0.79) | 0.87 (0.74-1.02) |
| Systemic corticosteroids | 1.17 (1.09-1.25) | 1.08 (0.97-1.21) | 1.08 (0.95-1.22) | 1.15 (1.00-1.33) |
| Pulmonary medications | 1.01 (0.94-1.09) | 1.03 (0.92-1.15) | 1.00 (0.87-1.14) | 0.90 (0.78-1.05) |
| Vasopressors | 2.21 (1.98-2.48) | 3.47 (2.80-4.31) | 2.78 (2.11-3.66) | 1.59 (1.37-1.85) |
| Mechanical ventilation | ||||
| Invasive | 1.17 (1.05-1.31) | 3.91 (3.05-5.01) | 1.36 (0.84-2.20) | 0.94 (0.82-1.08) |
| Noninvasive | 1.44 (1.27-1.62) | 2.09 (1.67-2.61) | 1.70 (1.25-2.28) | 1.10 (0.94-1.29) |
| Organ failure | ||||
| Respiratory | 2.88 (2.64-3.14) | NA | NA | 0.83 (0.62-1.12) |
| Cardiovascular | 1.67 (1.51-1.85) | 1.58 (1.34-1.86) | 1.56 (1.28-1.91) | 1.81 (1.53-2.14) |
| Neurologic | 1.45 (1.23-1.71) | 1.39 (1.07-1.79) | 1.76 (1.26-2.37) | 1.21 (0.91-1.62) |
| Renal | 1.59 (1.48-1.72) | 1.88 (1.68-2.09) | 1.50 (1.30-1.74) | 1.32 (1.15-1.52) |
| Hematologic | 1.64 (1.43-1.90) | 1.57 (1.25-1.96) | 1.31 (0.98-1.73) | 2.05 (1.58-2.66) |
| Hepatic | 2.84 (1.84-4.37) | 3.48 (1.75-6.64) | 2.34 (1.02-5.17) | 2.48 (1.15-5.72) |
| Antibiotic therapy | ||||
| GC-CAP therapy | 0.40 (0.38-0.43) | 0.42 (0.38-0.46) | 0.48 (0.43-0.54) | 0.51 (0.45-0.58) |
| MRSA therapy | 1.13 (1.06-1.21) | 1.25 (1.13-1.37) | 1.12 (1.00-1.26) | 0.81 (0.69-0.93) |
Data are adjusted odds ratios (95% confidence intervals). Odds ratios > 1 indicate an increased risk of 30-day mortality, whereas odds ratios < 1 indicate a decreased risk of 30-day mortality.
COPD: chronic obstructive pulmonary disease; GC-CAP: guideline-concordant community-acquired pneumonia.
Race was ordered as black vs. nonblack.
Collinearity was determined through theoretical relations for select variables. Variables suspected of being collinear were excluded from the models. For instance, most patients receiving hemodialysis also had renal failure; therefore, renal failure was chosen as the variable for the models, and the hemodialysis variable was excluded. The Charlson score and the “any organ failure” variables were excluded from the models because individual comorbidities and organ failures were already included in the models. Individual risk score variables were also excluded from the models because our study ran separate multivariable models for the three risk groups, and these individual characteristics were used to define those risk groups.
The final list of covariates included the following: patient age, race, Hispanic ethnicity, myocardial infarction, heart failure, chronic obstructive pulmonary disease, liver disease, renal disease, diabetes mellitus, neoplastic disease, cardiovascular medications, antidiabetic medications, inhaled corticosteroids, systemic corticosteroids, pulmonary medications, vasopressors, invasive and noninvasive mechanical ventilation, respiratory failure, cardiovascular failure, neurologic failure, renal failure, hematologic failure, hepatic failure, GC-CAP therapy, and MRSA therapy.
Results
Overall Population
A total of 50,119 patients met our study criteria, of whom 62% received Pseudomonas therapy. Patients were predominantly elderly (median age 78 years), white (83%) men (98%). One or more HCAP criteria was the most common MDR risk score variable (36%), followed by hospitalization in the past 90 days (24%), respiratory organ failure (11%), and invasive mechanical ventilation (6%). Patients were classified as low (68%), medium (21%), or high (11%) risk of drug-resistant pathogens.
The median (IQR) Charlson score was 2 (1-4), and common comorbidities included chronic obstructive pulmonary disease (53%), diabetes (33%), heart failure (26%), and neoplastic disease (26%). The most commonly used medications within 90 days prior to admission included cardiovascular medications (71%) and pulmonary medications (39%). Organ failure occurred in 24% of patients. Finally, 80% of patients received GC-CAP therapy, and 34% received MRSA therapy.
Baseline Characteristics
Many statistically significant differences were observed between patients who received Pseudomonas therapy and those who did not receive Pseudomonas therapy (Table 2); however, not more than a 3% difference between groups was noted for most of the comparisons, so many of these differences are probably not clinically meaningful. Notable differences were that patients who received Pseudomonas therapy were significantly more likely to have an HCAP risk factor, have been hospitalized in the last 90 days, have received cardiovascular medications prior to admission, and have received GC-CAP therapy and MRSA therapy during their hospital stay.
Patient Mortality
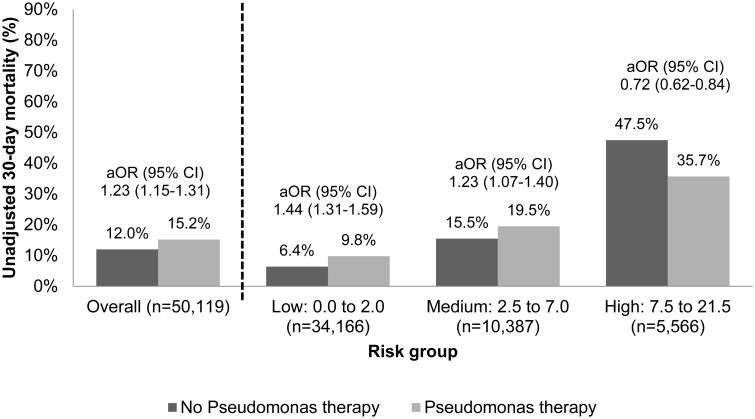
The overall 30-day mortality rate was 14%, and the unadjusted 30-day mortality increased significantly from the low-risk (8%) and medium-risk (18%) groups to the high-risk group (40%). This increase in patient mortality with increase in risk score was consistent, regardless of whether we used the numeric risk scores or the risk score categories. Pseudomonas therapy (aOR 0.72, 95% CI 0.62-0.84) was significantly associated with lower 30-day mortality in the high-risk group but not in the low or medium-risk groups (Figure 1). The complete multivariable models are shown in Table 3.
Figure 1.
Thirty-day mortality stratified by risk group (low, medium, and high) and Pseudomonas therapy versus no Pseudomonas therapy groups within each risk group. aOR = adjusted odds ratio; CI = confidence interval.
Discussion
Despite the various pneumonia assessment tools available, an efficient approach to identify appropriate candidates for empiric Pseudomonas therapy at hospital admission is still lacking. Although generally regarded as a nosocomial pathogen, Pseudomonas is a clinically significant, albeit infrequent, source of CO-pneumonia. Optimizing initial empiric antimicrobial therapy for Pseudomonas CO-pneumonia is critical, given its known propensity to harbor and acquire multidrug-resistance determinants. However, empiric regimens targeting the more prevalent CO-pneumonia pathogens, such as S. pneumoniae, may not provide sufficient microbiologic activity against Pseudomonas. Clinicians need strategies to identify patients who can benefit from empiric Pseudomonas therapy while keeping a more conservative approach for those who are at low risk. Ma et al. developed a risk score to identify patients with pneumonia at risk for drug-resistant pathogens.17 Using a similar risk score, our study demonstrated a survival advantage among high-risk patients who received empiric Pseudomonas therapy within 48 hours of hospital admission. This survival advantage was not present for patients in the low- and medium-risk groups.
Our study supports the notion that this new risk score might be a better alternative to guide empiric Pseudomonas therapy in patients with CO-pneumonia than the HCAP criteria.3 Approximately 36% of this study population fulfilled criteria for HCAP, whereas only 11% of the Pseudomonas therapy group were classified as having high-risk for drug-resistant pathogens. The application of the HCAP criteria may have led to overuse of broad-spectrum antibiotics among patients with lower risk scores who may be less likely to benefit from empiric antipseudomonal therapy. Several studies have demonstrated that the HCAP criteria have low specificity for Pseudomonas pneumonia and that grouping risk factors for MRSA with other gram-negative, drug-resistant pathogens may lead to inappropriate broad-spectrum antibiotic treatment.26-30 Recently, Chalmers et al. conducted a meta-analysis of 24 studies that compared HCAP and CAP cohorts. The study concluded that the ability for the HCAP criteria to appropriately identify patients with drug-resistant pathogens, including Pseudomonas, was low and did not meet the threshold for clinical use.31 In addition, most studies evaluating the utility of HCAP criteria to guide broad-spectrum therapy, including Pseudomonas therapy, have not demonstrated improved mortality with guideline-recommended therapies.15,18,19,32,33
Maruyama et al. conducted a prospective clinical trial in patients with HCAP and then considered severity of illness and number of MDR risk factors for each patient to decide which patients should receive Pseudomonas therapy.34 Those with nonsevere disease and ≥2 MDR risk factors, and those with severe disease and ≥1 MDR risk factor, received Pseudomonas therapy with an antipseudomonal β-lactam plus a fluoroquinolone or aminoglycoside, plus optional linezolid or vancomycin. Other patients received only CAP therapy. The authors stated that their patients achieved good outcomes without excessive use of broad-spectrum therapy. However, without a comparison group, it is difficult to tell how these patients might have fared if they had not received Pseudomonas coverage. Our study adds an important additional piece of information to the existing literature; it is a study of patients with MDR risk factors with an important comparison of those who received and did not receive Pseudomonas therapy.
One advantage of the risk score by Ma et al17 is the weighting of important risk factors. Similar to Ma's scoring system, we assigned more weight to patients with respiratory failure (14 points) and recent hospitalization (5 points) compared to patients with other risk factors such as invasive mechanical ventilation (2 points) or HCAP risk factors (0.5 point). This stratification highlights a key feature of the risk score because patients with more serious risk factors, such as respiratory failure or recent hospital admission, cannot be classified into the low-risk group. One potential limitation of the rule is that it assigns 5 points for hospitalization within the last 90 days and 0.5 point for any HCAP risk factor, including recent hospitalization. We maintained that strategy in our study, to keep our rule as consistent with the one by Ma et al17 as possible; however, this is a potential limitation of both studies.
Our own study has other limitations. First, we used the rule developed by Ma et al17 but we acknowledge that other rules have been developed to predict which patients likely have MDR pathogens and/or Pseudomonas aeruginosa.3,14,26,28-30,33,35-38 There are two reviews that have compared six and nine of these MDR prediction rules, respectively.32,39 Both reviews concluded that none of the examined rules worked well enough to identify patients with MDR pathogens. It is important to note that the rule by Ma et al17 was not part of either review, so it is unknown how it performs when compared to the rules previously studied. Most importantly, this is the first study that has attempted to investigate whether patients deemed high risk by a hypothesized rule fare better when given broad-spectrum antibiotics. None of the previously studied rules, or other follow-up studies, have closely investigated this relationship. This is the key idea advanced by the present study.
Other MDR prediction rules and prior studies have identified risk factors associated with Pseudomonas pneumonia that were not included in the risk score used for our study. These include bronchiectasis,17,40 chronic obstructive pulmonary disease,2,9,41 enteral tube feedings,13 and previous colonization with Pseudomonas.11,42 Similarly, there are patient and provider characteristics that were not included in our analyses that might affect patient outcomes. These include specific antimicrobial medications and doses received, nonantimicrobial medications, pathogens, antimicrobial susceptibilities, patient functional status and clinical presentation, and provider preferences. Our changes to the variable definitions from the study by Ma et al17 might also have affected outcomes. We had to change the bronchiectasis variable (14 points) to a respiratory organ failure variable (14 points) because the presence or absence of bronchiectasis was not available in our database. We changed the severe pneumonia variable (2 points) to an invasive mechanical ventilation variable (2 points) because our database did not contain CURB-65 scores. This likely resulted in a slightly different population of patients who met our modified rule as compared to the rule published by Ma et al.17 In addition, Ma et al17 derived the risk score based on an elderly population in Hong Kong where there may be significant differences in risk factors, prevalence of drug-resistant pathogens, and susceptibility profiles. Also, ICD-9-CM codes were used to identify patients with pneumonia and baseline characteristics. This approach could potentially lead to misclassification bias or underestimate the true prevalence of the pathogens, and cannot be considered equivalent to a medical chart review.
Our study population of older adult males (all ≥ 65 years of age and 98% male) does not reflect the usual patient mix found in non-VA hospitals. Therefore, the findings of this study may not be generalizable to all settings. Also, the study by Ma et al17 used a clinical definition of pneumonia, whereas our study used administrative codes to define pneumonia. Furthermore, it is possible that patients in the low-risk group who received antipseudomonal therapy did worse because of the greater prevalence of some other problematic pathogen for which antipseudomonal antibiotics have no activity, and/or the inability to account for other risk factors associated with infection severity and outcomes not measured in this study.
Finally, we believe that the MDR risk score evaluated in this study can be used to identify patients who might benefit from Pseudomonas therapy. We also believe that a different MRSA risk score can be used to identify patients who might benefit from MRSA therapy.43 In other words, we believe that different sets of risk factors should be used to determine which patients should receive MRSA and Pseudomonas therapy. This is consistent with the new approach to HCAP suggested by the 2016 HAP/VAP guidelines.4 These new guidelines suggest that clinicians use validated risk factors for pathogens, rather than solely relying on HCAP risk factors, when deciding which patients should receive different therapies.4 That said, we recognize that many patients will have risk factors that warrant both MRSA and Pseudomonas therapies.
Conclusion
This study demonstrated that use of a risk score can be applied at hospital admission to identify patients who are likely to benefit from empiric Pseudomonas therapy. Widespread use of this score could reduce overuse of anti-Pseudomonas antibiotics in low- to medium-risk patients and improve survival in high-risk patients.
Acknowledgments
Dr. Mortensen was supported in part by the University of Texas Southwestern Center for Patient-Centered Outcomes Research. This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
Funding: This work was supported by the National Institutes of Health (R01 NR010828 to E.M.M. and KL2 RR025766, KL2 TR000118 to C.R.F.) and the Agency for Healthcare Research and Quality (R24 HS022418 to E.M.M.). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: Dr. Frei has received research grants to his institution for investigator-initiated cancer and infectious diseases research, and from Bristol-Myers Squibb, Forest, Pfizer, and Pharmacyclics in the past 3 years.
References
- 1.Heron M. Deaths: Leading Causes for 2011. Natl Vital Stat Rep. 2015;64:1–96. [PubMed] [Google Scholar]
- 2.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG Infectious Diseases Society of A, American Thoracic S. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–62. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 6.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–73. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers JD, Taylor JK, Singanayagam A, Fleming GB, Akram AR, Mandal P, Choudhury G, Hill AT. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis. 2011;53:107–13. doi: 10.1093/cid/cir274. [DOI] [PubMed] [Google Scholar]
- 8.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–19. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 9.Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, Torres A. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162:1849–58. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 10.Rello J, Bodi M, Mariscal D, Navarro M, Diaz E, Gallego M, Valles J. Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest. 2003;123:174–80. doi: 10.1378/chest.123.1.174. [DOI] [PubMed] [Google Scholar]
- 11.Gross AE, Van Schooneveld TC, Olsen KM, Rupp ME, Bui TH, Forsung E, Kalil AC. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58:5262–8. doi: 10.1128/AAC.02582-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khawaja A, Zubairi AB, Durrani FK, Zafar A. Etiology and outcome of severe community acquired pneumonia in immunocompetent adults. BMC Infect Dis. 2013;13:94. doi: 10.1186/1471-2334-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Baum H, Welte T, Marre R, Suttorp N, Ewig S group Cs. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: Diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 14.Park SC, Kang YA, Park BH, Kim EY, Park MS, Kim YS, Kim SK, Chang J, Jung JY. Poor prediction of potentially drug-resistant pathogens using current criteria of health care-associated pneumonia. Respir Med. 2012;106:1311–9. doi: 10.1016/j.rmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Falcone M, Russo A, Giannella M, Cangemi R, Scarpellini MG, Bertazzoni G, Alarcon JM, Taliani G, Palange P, Farcomeni A, Vestri A, Bouza E, Violi F, Venditti M. Individualizing risk of multidrug-resistant pathogens in community-onset pneumonia. PLoS One. 2015;10:e0119528. doi: 10.1371/journal.pone.0119528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huttner B, Jones M, Madaras-Kelly K, Neuhauser MM, Rubin MA, Goetz MB, Samore MH. Initiation and termination of antibiotic regimens in Veterans Affairs hospitals. J Antimicrob Chemother. 2015;70:598–601. doi: 10.1093/jac/dku388. [DOI] [PubMed] [Google Scholar]
- 17.Ma HM, Ip M, Woo J, Hui DS. Development and validation of a clinical risk score for predicting drug-resistant bacterial pneumonia in older Chinese patients. Respirology. 2014;19:549–55. doi: 10.1111/resp.12267. [DOI] [PubMed] [Google Scholar]
- 18.Attridge RT, Frei CR, Restrepo MI, Lawson KA, Ryan L, Pugh MJ, Anzueto A, Mortensen EM. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38:878–87. doi: 10.1183/09031936.00141110. [DOI] [PubMed] [Google Scholar]
- 19.Grenier C, Pepin J, Nault V, Howson J, Fournier X, Poirier MS, Cabana J, Craig C, Beaudoin M, Valiquette L. Impact of guideline-consistent therapy on outcome of patients with healthcare-associated and community-acquired pneumonia. J Antimicrob Chemother. 2011;66:1617–24. doi: 10.1093/jac/dkr176. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, Good C, Restrepo MI, Downs JR, Frei CR, Fine MJ. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55:1466–73. doi: 10.1093/cid/cis733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen EM, Halm EA, Pugh MJ, Copeland LA, Metersky M, Fine MJ, Johnson CS, Alvarez CA, Frei CR, Good C, Restrepo MI, Downs JR, Anzueto A. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311:2199–208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen EM, Coley CM, Singer DE, Marrie TJ, Obrosky DS, Kapoor WN, Fine MJ. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162:1059–64. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 25.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22:316–25. doi: 10.1097/QCO.0b013e328329fa4e. [DOI] [PubMed] [Google Scholar]
- 27.Ewig S, Welte T, Torres A. Is healthcare-associated pneumonia a distinct entity needing specific therapy? Curr Opin Infect Dis. 2012;25:166–75. doi: 10.1097/QCO.0b013e32835023fb. [DOI] [PubMed] [Google Scholar]
- 28.Shorr AF, Zilberberg MD, Reichley R, Kan J, Hoban A, Hoffman J, Micek ST, Kollef MH. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis. 2012;54:193–8. doi: 10.1093/cid/cir813. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber MP, Chan CM, Shorr AF. Resistant pathogens in nonnosocomial pneumonia and respiratory failure: is it time to refine the definition of health-care-associated pneumonia? Chest. 2010;137:1283–8. doi: 10.1378/chest.09-2434. [DOI] [PubMed] [Google Scholar]
- 30.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med. 2008;168:2205–10. doi: 10.1001/archinte.168.20.2205. [DOI] [PubMed] [Google Scholar]
- 31.Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:330–9. doi: 10.1093/cid/cit734. [DOI] [PubMed] [Google Scholar]
- 32.Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109:1–10. doi: 10.1016/j.rmed.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Madaras-Kelly KJ, Remington RE, Fan VS, Sloan KL. Predicting antibiotic resistance to community-acquired pneumonia antibiotics in culture-positive patients with healthcare-associated pneumonia. J Hosp Med. 2012;7:195–202. doi: 10.1002/jhm.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruyama T, Fujisawa T, Okuno M, Toyoshima H, Tsutsui K, Maeda H, Yuda H, Yoshida M, Kobayashi H, Taguchi O, Gabazza EC, Takei Y, Miyashita N, Ihara T, Brito V, Niederman MS. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57:1373–83. doi: 10.1093/cid/cit571. [DOI] [PubMed] [Google Scholar]
- 35.Aliberti S, Di Pasquale M, Zanaboni AM, Cosentini R, Brambilla AM, Seghezzi S, Tarsia P, Mantero M, Blasi F. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis. 2012;54:470–8. doi: 10.1093/cid/cir840. [DOI] [PubMed] [Google Scholar]
- 36.Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Goto Y, Fukui Y, Iwaki M, Okumura J, Yamaguchi I, Yagi T, Tanikawa Y, Sugino Y, Shindoh J, Ogasawara T, Nomura F, Saka H, Yamamoto M, Taniguchi H, Suzuki R, Saito H, Kawamura T, Hasegawa Y Central Japan Lung Study G. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: an observational cohort study. Lancet Infect Dis. 2015 doi: 10.1016/S1473-3099(15)00151-6. [DOI] [PubMed] [Google Scholar]
- 37.Kollef MH, Morrow LE, Baughman RP, Craven DE, McGowan JE, Jr, Micek ST, Niederman MS, Ost D, Paterson DL, Segreti J. Health care-associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes--proceedings of the HCAP Summit. Clin Infect Dis. 2008;46(4):S296–334. doi: 10.1086/526355. quiz 5-8. [DOI] [PubMed] [Google Scholar]
- 38.El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39:474–80. doi: 10.1086/422317. [DOI] [PubMed] [Google Scholar]
- 39.Self WH, Wunderink RG, Williams DJ, Barrett TW, Baughman AH, Grijalva CG. Comparison of clinical prediction models for resistant bacteria in community-onset pneumonia. Acad Emerg Med. 2015;22:730–40. doi: 10.1111/acem.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polverino E, Cilloniz C, Menendez R, Gabarrus A, Rosales-Mayor E, Alcaraz V, Terraneo S, Puig de la Bella Casa J, Mensa J, Ferrer M, Torres A. Microbiology and outcomes of community acquired pneumonia in non cystic-fibrosis bronchiectasis patients. J Infect. 2015;71:28–36. doi: 10.1016/j.jinf.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Rello J, Rodriguez A, Torres A, Roig J, Sole-Violan J, Garnacho-Montero J, de la Torre MV, Sirvent JM, Bodi M. Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J. 2006;27:1210–6. doi: 10.1183/09031936.06.00139305. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Zorrilla S, Camoez M, Tubau F, Canizares R, Periche E, Dominguez MA, Ariza J, Pena C. Ability to develop Pseudomonas aeruginosa clinical infections according to the prior rectal colonization status: a prospective observational study. Antimicrob Agents Chemother. 2015;59:5213–9. doi: 10.1128/AAC.04636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teshome BF, Lee GC, Reveles KR, Attridge RT, Koeller J, Wang CP, Mortensen EM, Frei CR. Application of a methicillin-resistant Staphylococcus aureus risk score for community-onset pneumonia patients and outcomes with initial treatment. BMC Infect Dis. 2015;15:380. doi: 10.1186/s12879-015-1119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]