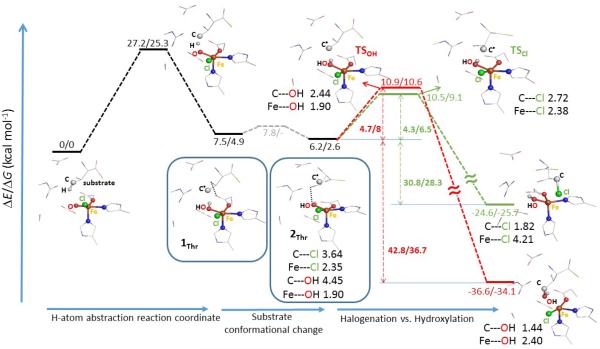
Figure 1.
Chlorination vs. hydroxylation (in green vs. red) from the Cl—FeIII(S=5/2)—OH product of the H-atom abstraction pathway (black), proceeding via the Cl—FeIV=O π-attack on the native substrate L-Thr. The relative potential energies and Gibbs free energies are in kcal mol−1, the key geometric parameters are in Å. Note that the water molecule present in the cluster model (from Figure 9A in ref 8) is not depicted. The relative enthalpies are provided in Figure S2. For detailed comparison of two substrate-radical conformations 1Thr vs. 2Thr see Figure S3A. The dependence of equilibrium geometries on a basis set was tested by performing geometry optimizations for 2Thr and the related transition states for chlorination and hydroxylation, using a combined basis set (def2-TZVP for Fe, ligating atoms and the terminal C atom of the substrate; def2-SVP for the rest). In comparison with the def2-SVP equilibrium geometries, the geometric changes are small, which translates into small changes of calculated reaction energies (c.f. 4.5 and 5.1 kcal mol−1 for chlorination and hydroxylation using the hybrid basis set for geometry optimizations vs. 4.3 and 4.7 kcal mol−1 with the def2-SVP equilibrium geometries).