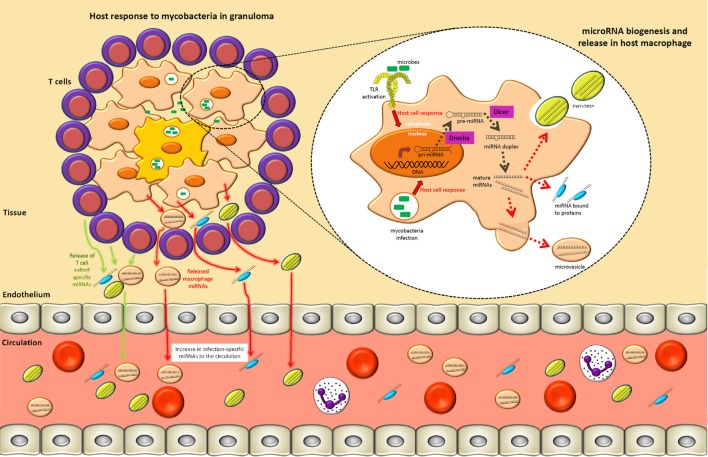
Figure 1.
A tuberculosis lung granuloma demonstrates how specific circulating microRNAs (miRNAs) may arise during an infection process. Mycobacterial pathogen-associated molecular patterns are recognized by toll-like receptors (TLRs) and other pattern recognition receptors, which result in the upregulation of primary-miRNAs in macrophages. These transcripts are subsequently cleaved in the nucleus and cytoplasm by Drosha and Dicer, respectively, resulting in 21–25 nucleotide mature miRNAs that act to fine-tune intracellular immune processes. Specific pathways and components of the immune response may be regulated by different miRNA subsets. Concurrently, the surrounding T lymphocytes involved in granuloma formation/maintenance upregulate T cell subset-specific miRNAs as a means of modulating the type of adaptive immune response. Mature miRNAs generated in macrophages and T cells may also be released into the extracellular environment within exosomes, heterogeneous microvesicles, or in association with high-density lipoprotein, LDL, or other protein complexes. Subsequently, by means not yet fully understood, these extracellular miRNAs move from local sites of infection to the circulatory system. This process can therefore give rise to infection-specific circulating miRNA expression signatures that can readily be accessed from multiple biological fluids (e.g., serum, plasma, or sputum).