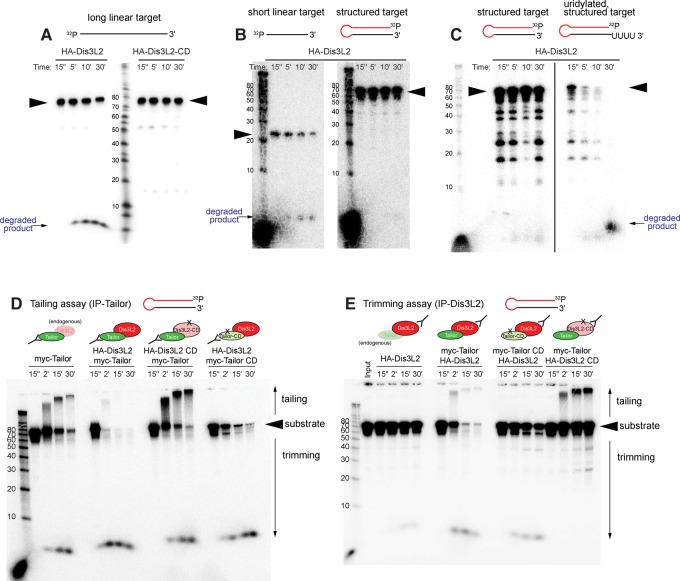
FIGURE 3.
Tailor-mediated uridylation enhances Dis3L2-mediated degradation of structured substrates. The in vitro tests shown here assess the capacity of immunoprecipitated factors to modify different 5′ radiolabeled substrates, as assayed on a reaction timecourse. (A) Parallel assays of HA-Dis3L2-IP and HA-Dis3L2-CD-IP material on an artificial linear target demonstrate exoribonuclease activity of Dis3L2. The input material (arrowhead) is decreased upon incubation with HA-Dis3L2, concomitant with accumulation of shorter species, but not with HA-Dis3L2-CD. (B) Parallel assays of HA-Dis3L2 on the model substrates’ mature miR-1010 (short linear target) and pre-mir-1010 (structured target); the miR-1010 sequence comprises the 3′ terminal 24 nt of the pre-mir-1010 hairpin. Dis3L2 is less effective at degrading the structured target. (C) Parallel assays of HA-Dis3L2 on normal and uridylated pre-mir-1010 shows that degradation of this hairpin is facilitated by a short uridine tail. (D) Tailing assays (i.e., interrogating Tailor-IP material) from cells cotransfected with combinations of wild-type and catalytically dead Tailor or Dis3L2 assayed on pre-mir-1010. Tailor-IP causes substrate tailing, while Tailor-IP from cells cotransfected with Tailor and Dis3L2 mediates robust substrate degradation. This is dependent on Dis3L2 catalytic status, and partially dependent on Tailor catalytic status. Substrate decay in the presence of Dis3L2-CD may potentially be due to endogenous Dis3L2. (E) Trimming assays (i.e., interrogating Dis3L2-IP material) from cells cotransfected with combinations of wild-type and catalytically dead Tailor or Dis3L2 assayed on pre-mir-1010. Substrate degradation by Dis3L2-IP is very strongly potentiated by cotransfection with Tailor. This is dependent on the catalytic status of Tailor and Dis3L2.