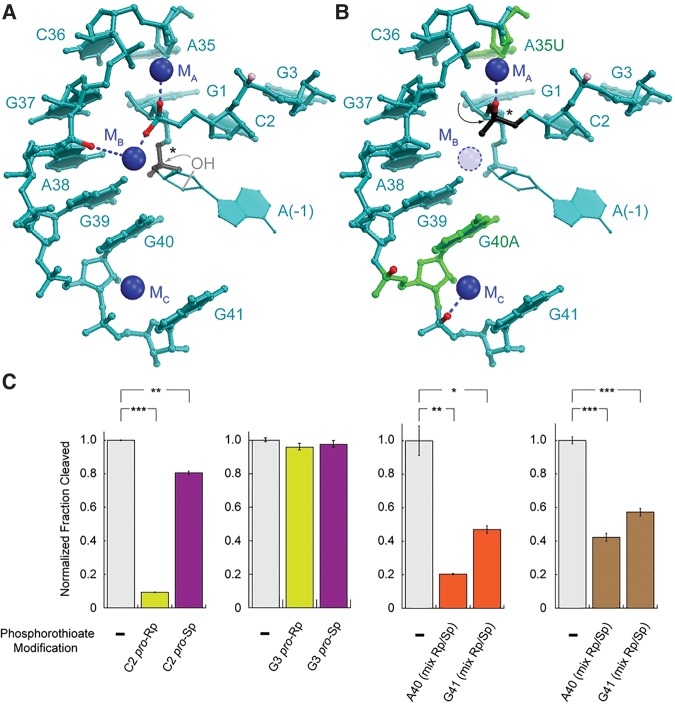
FIGURE 8.
glmSCa co-opts a preexisting structural cation for activity. (A) The glmSAAA active site (Lau and Ferré-D'Amaré 2013). NBPOs with strong and moderate phosphorothioate interference are in red and pink, respectively. Scissile phosphate is in black (*). Residue (−1) is modeled (Lau and Ferré-D'Amaré 2013). (B) Phosphorothioate interferences in glmSCa mapped onto the glmSAAA structure. Residues that differ between glmSWT and glmSCa are in green. (C) Effect of NBPO sulfur substitutions on glmSCa. Substitutions of G3 serve as a quality control on the assembly of phosphorothioate-containing ribozymes. Activities of unsubstituted glmSCa, pro-Rp, and pro-Sp NBPO substitutions, and diastereomeric substitutions are in gray, yellow, purple, and orange, respectively. Sr2+ activities are shown in brown. (*), (**), and (***) denote 0.05 < P < 0.01, 0.001 < P < 0.01, and P < 0.001, respectively (two-tailed t-test).