Abstract
Background
Understanding the prevalence of soil-transmitted helminth infections is necessary to plan control strategies and focus on highly endemic regions for preventive chemotherapy and improved sanitation facilities. India is known to be endemic for soil-transmitted helminth infections.
Methods
To understand the prevalence, spatial distribution and identify high-risk zones, a systematic search of published literature was carried out based on PRISMA guidelines from the year 2000 to 2015.
Results
A careful screening of the identified literature yielded 39 studies that reported the prevalence of soil-transmitted helminth infections from 19 different states of India. Ascaris lumbricoides was the most prevalent parasite. Higher than 50% prevalence was reported from six states. Nearly 90% studies reported the prevalence of more than one parasite species in the same sample population.
Conclusion
This is the first study to comprehensively review the literature associated with soil-transmitted helminth infections from India giving a clear idea of its prevalence, distribution and high endemic areas.
Background
Soil-transmitted Helminths (STH) infects nearly 2 billion people of world’s population with children being the most affected [1]. According to the World Health Organization (WHO) estimates, 870 million children live in the area of high prevalence. Africa, South Asia and South America are the most affected regions of the world [2]. India alone contributes nearly 25% to the total global cases with 220.6 million children in need of preventive chemotherapy [3]. STH infections rarely cause mortality with diarrhea, abdominal pain and low hemoglobin levels as the immediate outcome of infections, however, the long term effects of these infections are far more sinister as those with infections show reduced cognitive abilities, intellectual capacity and lower work productivity [4]. The warm and moist climate of tropical and subtropical countries provides the ideal environment for the survival of parasite eggs or larvae of these four STH, roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura) and hookworm (Necator americanus, Ancylostoma duodenale) [5].
The prevalence and control of STH infections is inextricably linked with water quality, sanitation, hygiene practices and socio-economic status in the affected areas [6]. Despite the fact that infection can be cured with either Albendazole or Mebendazole, eradication is difficult, given STH’s feco-oral and penetration-via-skin transmission pattern as the chances of reinfection are very high in population living in affected areas [7]. Control is achieved by targeted use of chemotherapy and improvement of sanitation, drinking water, use of pit-latrines instead of open defecation and good hygiene practices [8–10]. India, with support from WHO, launched a Global Programme for the Elimination of Lymphatic Filariasis in the year 2000 (http://nvbdcp.gov.in/doc/guidelines-filariasis-elimination-india.pdf). Under this programme Diethylcarbamazine (DEC) and Albendazole were administered to people living in filarial endemic areas. In 2015 another nation-wide deworming programme was launched covering nearly 241 million children with STH infection or at risk of developing the infection (http://nrhm.gov.in/nrhm-components/rmnch-a/child-healthimmunization/national-deworming-day-february-2017.html). For such targeted efforts a comprehensive knowledge of the prevalence pattern of these helminth infections needs to be assessed thoroughly. Several studies have been published from India by clinicians and researchers assessing the prevalence and epidemiology of STH infections in different states and municipalities. Such studies have the potential to guide governmental and non-governmental organizations (NGOs) to focus their efforts in highly endemic areas. The purpose of this review is to evaluate the prevalence of STH infections and identify high-risk areas in different regions of India, based on a comprehensive search and analysis of published literature. Such an exercise would help Governmental agencies and NGOs to focus on specific areas of high prevalence for preventive chemotherapy and improved sanitation practices.
Methods
Search Strategy and Data extraction
We did a review based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to identify all relevant publications pertaining to the prevalence of STH infections in India. We systematically searched PubMed and Web of knowledge from January 1, 2000 to June 30, 2015. We did not search before the year 2000 because our goal was to inform decision-making rather than providing a historical perspective. In this regard, it is pertinent to mention that STH infections are often a function of sanitation, health care and economic condition. In the last few decades India has seen considerable improvement in sanitation with a reduction of 31% in open defection. Community based and school based mass deworming programs have been undertaken to reduce the burden of STH infections.
We used the following search terms anywhere in the articles: “soil transmitted helminth” or “ascaris” or “trichuris” or “whipworm” or “necator” or “ancylostoma” or “hookworm” AND “India”. We searched without any bar on language or nature of studies. To identify additional studies, reference lists of publications were carefully screened. Initial assessment was based on review of title and abstract of all the studies. Full text of potentially relevant studies was further analyzed for STH prevalence data.
STH infections are largely asymptomatic in nature, and it is this asymptomatic population that carries the heaviest burden of infection. Only acute cases are reported in hospitals. In this context, we included studies reporting prevalence data from community based cross-sectional studies only. Retrospective analysis and hospital based observational studies were excluded. Any study that did not report either number of participants or age group of target population or method of parasitological testing or geographical location or had discrepancy in what was reported in the abstract and full text were all excluded from the final analysis. Cross-sectional studies with full-text availability, and reporting prevalence of at least one parasite in a defined population (location, age group, number of participants) were included in the review. In case time period of the study was not specified, the date of publication was considered instead. If more than one publication was reported from the same geographical area, then most recent publication was considered for final analysis.
The data extracted from the selected publications included first author, date of publication, date of survey, state and localities where the study was carried out, sample size and age, type of parasitological testing performed, study design and percentage prevalence of each parasite. All the data was entered in an excel file and double-checked.
Prevalence mapping
For prevalence mapping, locations where the studies were carried out were georeferenced using Google maps. A total of 49 unique locations were identified and georeferenced from where A. lumbricoides infections were reported. 39 unique locations were identified for T. trichiura and 35 locations were identified for hookworm infections.
Results
Initial searches identified 480 studies from PubMed and web of knowledge. After removing duplicates and irrelevant articles 127 studies were considered for full text review. Studies were excluded for not reporting prevalence data of parasites (42), lack of full text availability (10), mode of data collection (23) and incomplete data (10). Two studies were excluded as they reported data from the same geographical area; in each case only recently conducted study was considered. One study has discrepancy in what it reported in its full text and abstract. A total of 39 studies were identified that reported the prevalence of at least one STH infection among humans in India (Fig. 1). All 39 studies were cross sectional in nature with one study reporting data from pregnant females. Most of the reported data are from northern, northeastern, western and southern region of India, with a general lack of publication from northwest and southwest regions of India. Several studies (85%) reported prevalence of STH infection only in children. Smallest sample size was 25 and largest sample size was 3706. A total of 25,754 stool samples were screened for the presence of STH infection. A combination of Saline and iodine wet mount, Kato-Katz technique, salt flotation, formol-ether concentration, mini-FLOTAC and zinc sulphate concentration techniques were used for parasite detection. A total of 21 studies reported prevalence data for all three parasitic infections, 13 studies reported prevalence data for at least two parasites and 5 studies reported data only for a single parasite (Table 1). The spatial distribution of STH was spread across whole of India with a total of 49 unique locations identified and georeferenced for the prevalence of A. lumbricoides (Fig. 2), 39 locations for T. trichiura (Fig. 3), and 35 locations for hookworm (Fig. 4). Prevalence of soil-transmitted helminths varied widely with A. lumbricoides infection ranging from 0.6 to 91%, T. trichiura ranging from 0.7 to 72% and hookworm ranging from 0.02 to 52%. A higher than 50% prevalence for A. lumbricoides was reported from 10 different locations scattered across six states, Jammu and Kashmir, Assam, Bihar, Tamil Nadu, West Bengal and Andhra Pradesh covering nearly 30% of India’s population (Table 2) [11–49].
Fig. 1.
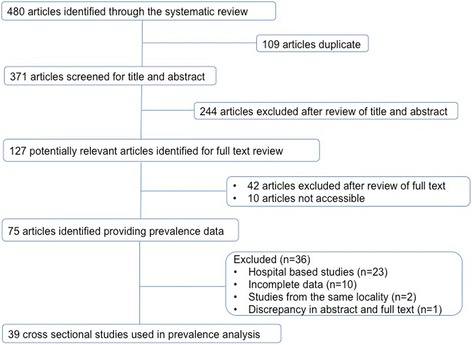
Schematic representation of the study selection process
Table 1.
Overview of the published reports considered for review
| Characteristic | n (%) |
|---|---|
| No. Of studies | 39 (100) |
| Studied Population | |
| Children | 33 (85) |
| Adult | 6 (15) |
| Parasite species reported | |
| All three | 21 (54) |
| A. lumbricoides + T. trichiura | 7 (18) |
| A. lumbricoides + Hookworm | 6 (15) |
| A. lumbricoides only | 4 (10) |
| Hookworm only | 1 (3) |
| Stool examination method | |
| Direct smear only | 9 (23) |
| Direct smear and/or salt-flotation, Zinc Sulphate flotation, Formalin-Ether concentration, mini-FLOTAC | 24 (62) |
| Kato-katz method | 6 (15) |
Fig. 2.
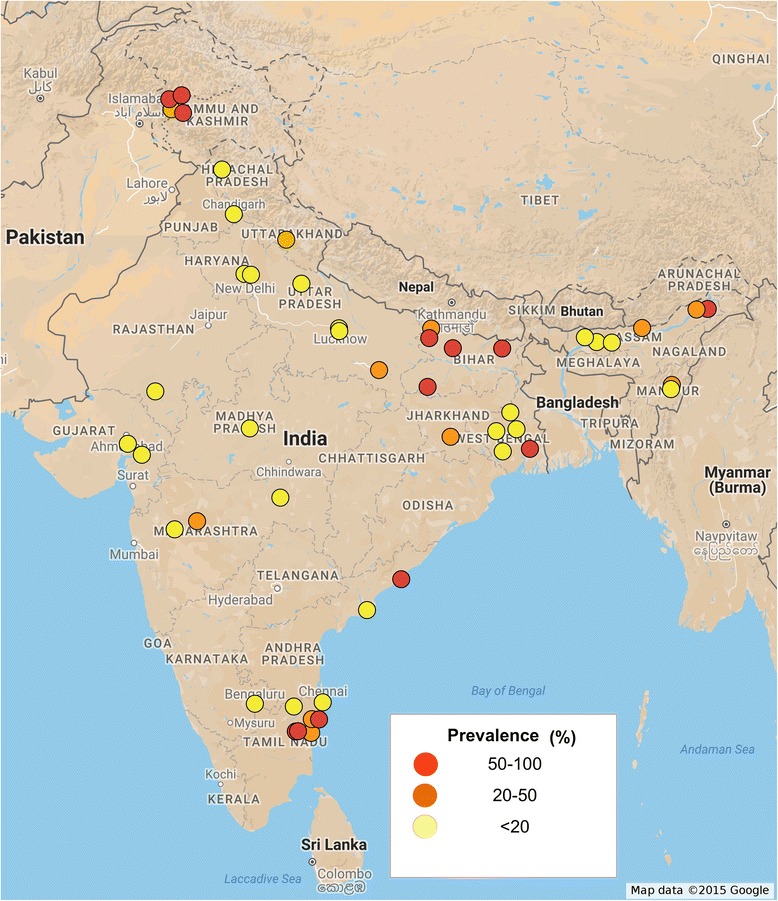
Spatial distribution of A. lumbricoides infections in India. Approval for reuse granted. (https://www.google.com/permissions/geoguidelines.html#general-guidelines)
Fig. 3.
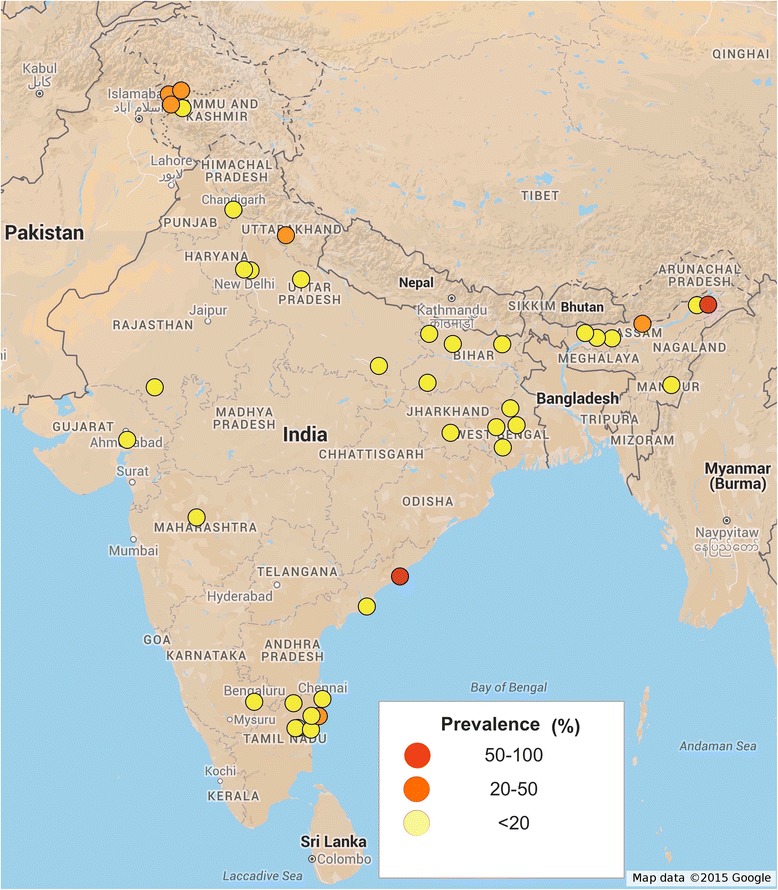
Spatial distribution of T. trichiura infections in India. Approval for reuse granted. (https://www.google.com/permissions/geoguidelines.html#general-guidelines)
Fig. 4.
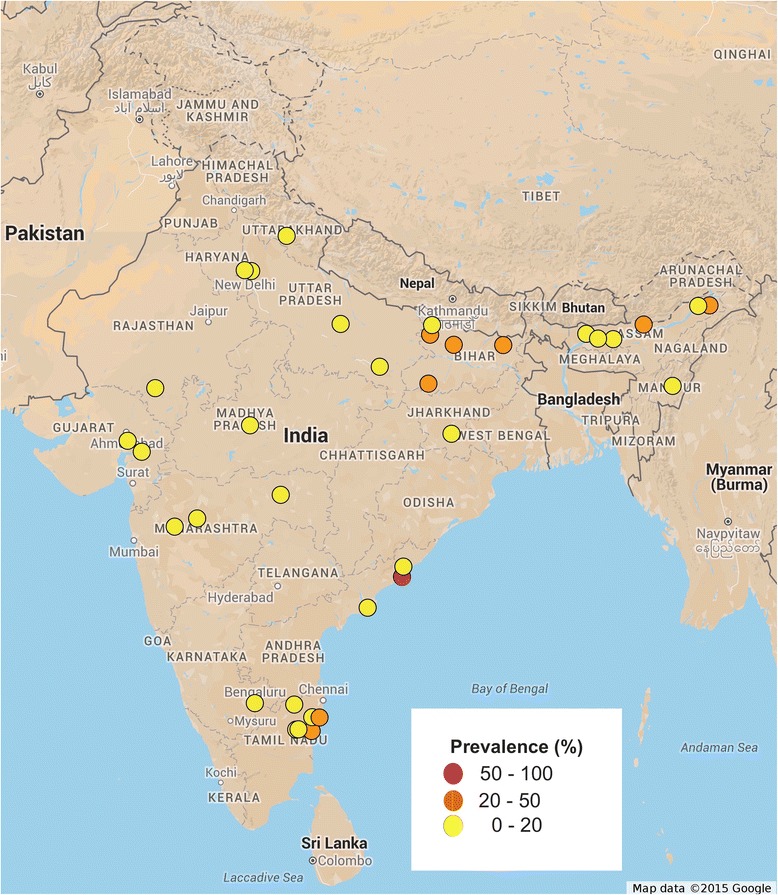
Spatial distribution of Hookworm infections in India. Approval for reuse granted. (https://www.google.com/permissions/geoguidelines.html#general-guidelines)
Table 2.
An overview of the extracted data considered for final analysis
| S.No. | Author [cite ID] Year | Study Design | Study Date | Location | Sample Size | Age Group | STH (%) | Technique | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AL | TT | HW | ||||||||
| Kashmir | ||||||||||
| 1 | Wani [11] 2008 |
Cross sectional | March-Nov. 2007 | Kashmir valley | 2256 | 0–15 | 68.3 | 27.92 | -- | Direct smear, zinc sulphate flotation, stolls egg counting technique. |
| 2 | Wani [12] 2010 |
Cross sectional | ND | Gurez valley | 352 | 1–15 | 71.18 | 26.42 | -- | Kato-Katz thick smear technique |
| 3 | Wani [13] 2007 |
Cross sectional | ND | Kupwara | 312 | 4–15 | 69.23 | 30.76 | -- | Direct smear and zinc sulphate flotation concentration |
| 4 | Singh [14] 2010 |
Cross sectional | Jan 2002 to Dec 2003 | Srinagar | 514 | 5–14 | 28 | 5 | -- | Iodine staining, Saline or zinc sulphate centrifugal floatation or sedimentation techniques |
| Delhi | ||||||||||
| 5 | Ranjan [15] 2013 |
Cross sectional | Nov-Jan. | Civil lines zone | 347 | 5–15 | 8.1 | 3.7 | 3.7 | Semi quantitative katokatz technique |
| Chandigarh | ||||||||||
| 6 | Sehgal [16] 2010 |
Cross sectional | Oct-Nov 2007 | Urban slums | 87 | Pregnant women | 3.4 | -- | -- | Direct wet smear |
| Uttarakhand | ||||||||||
| 7 | Bora [17] 2006 |
Cross sectional | August 2005 | Pauri Garhwal, | 257 | 9–10 | 28.8 | 1.9 | 5.1 | Kato-katz technique |
| Himachal Pradesh | ||||||||||
| 8 | Barda [18] 2013 |
Cross srctional | Jan-Apr 2012 | Dharamshala | 80 | 11–15 | 11.3 | Direct smear, Formolether concentration, mini-FLOTAC | ||
| Uttar Pradesh | ||||||||||
| 9 | Bisht [19] 2011 |
Cross sectional | June 2008 – Dec. 2009 | Ghaziabad | 335 | <14 | 2.4 | 1.8 | 1.2 | Normal saline, lugol iodine |
| 10 | Rashid [20] 2011 |
Cross sectional | Jan.-June 2010 | Bareilly | 320 | 5–16 | 9.68 | 1.56 | Direct wet smear examination | |
| 11 | Nitin [21] 2007 |
Cross sectional | May 2004-May 2006 | Lucknow Alambagh, Mati | 1071 | 1.5–85 | A-0.6 M-2.4 |
-- | M-0.7 | Wet mount examination |
| 12 | Awasthi [22] 2008 |
Cross sectional | July-Sept.2007 | Gyanpur | 909 | 14.56 months mean age | 41.9 | 2.6 | 6.1 | Formol ether |
| Madhya Pradesh | ||||||||||
| 13 | Tripathi [23] 2014 |
Cross sectional | July-Aug 2013 | Kajli Kheda, Bhopal | 300 | 6–12 | 4 | -- | 0.02 | Saline wet mount, Iodine wet mount |
| Rajasthan | ||||||||||
| 14 | Choubisa [24] 2012 |
Cross sectional | Oct.2010-Sep. 2011 | Udaipur | 224 | Below 60 | 4.46 | 0.44 | 0.89 | Lugol iodine, formol ether concentration |
| Gujarat | ||||||||||
| 15 | Shobha [25] 2013 |
Cross sectional | Feb-Dec 2008 | Urban slum, Baroda | 880 | <1 to >25 | 2.04 | -- | 0.11 | Wet smear (saline mount) examination and iodine mount. Formalin—ether sedimentation concentration |
| 16 | Lakhani [26] 2013 |
Cross sectional | Jun-Oct | Piparia Village, Vadodara District | 140 | 6–12 | 3.6 | 0.7 | 3.6 | Saline wet mounts as well as Lugol’s Iodine wet mounts, Formol ether concentration technique |
| Bihar | ||||||||||
| 17 | Pandey [27] 2013 |
Cross sectional | ND | Terai belt | 404 | 5–65 | 45.79 | -- | 8.16 | Saline and iodine wet mount |
| 18 | Greenland [28] 2015 |
Cross sectional | Jan-Feb 2011 | Araria, Aurangabad, Muzaffarpur and Gopalganj | 1157 | 4–17 | 51.9 | 4.7 | 41.8 | Kato-Katz smear |
| Jharkhand | ||||||||||
| Awasthi [22] 2008 |
Cross sectional | July-Sept. 2007 | Murhu | 909 | 14.56 months mean age | 22.3 | 2.6 | 1.5 | Formol ether | |
| Assam | ||||||||||
| 19 | Narain [29] 2004 |
Cross sectional | ND | Upper Assam | 265 | 2-all ages | 55 | 59 | 45 | Formol-ether concentration method |
| 20 | Traub [30] 2004 |
Cross sectional | July-Sept. 2000 | Phulbari Addabarie Balipara |
328 | 0–60 year | 38 | 43 | 43 | Water sedimentation technique followed by zinc sulphate centrifugation, katokatz technique |
| 21 | These [31] 2009 |
Cross sectional | Feb-Sept 2008 | Dibrugarh | 1029 | 5 to 13 | 63 | 19 | 1.7 | Direct and formal ether concentration methods |
| 22 | Sharma [32] 2012 |
Cross sectional | ND | Barpeta, Bongaigaon, Kamrup, Dibrugarh |
2499 | Adolescent girls, Mean 13.6 ± 1.1 |
10.6 | 6.2 | 3.9 | Formalin-ether method |
| Manipur | ||||||||||
| 23 | Singh [33] 2004 |
Cross sectional | Sept. 1998-October 2000 | Urban and rural areas | 1010 | 5–10 | 19.6 | 2.18 | 0.09 | Wet film, iodine preparation, formol ether techniques |
| 24 | Pukhrambam [34] 2013 |
Cross sectional | May-Jul 2012 | Urban slum (Hatta, Golapatti) | 255 | 1–5 | 21.5 | -- | -- | Saline and iodine mount method |
| Maharashtra | ||||||||||
| 25 | Dambhare [35] 2010 |
Cross sectional | Oct.-Nov. 2009 | Wardha (Karanji kaji village) | 172 | 6–14 | 0.6 | -- | 1.2 | Iodine staining of wet mounts |
| 26 | Aher [36] 2012 |
Cross sectional | ND | Ahmadnagar, Loni | 624 | 6–12 years. | 1.9 | -- | 0.9 | Saline and iodine preparation |
| 27 | Avhad [37] 2012 |
Cross sectional | June 2011-april 2012 | Aurangabad | 547 | 9–10 | 22.85 | 10.05 | 1.1 | Kato-katz technique |
| West Bengal | ||||||||||
| 28 | Mukherjee [38] 2013 |
Cross sectional | ND | Sunderbans Bankura, birbhum Midnapore, Bardhman Kolkata |
1192 | 10–15 | 11 | 1.8 | -- | Kato-katz technique |
| 29 | Sur [39] 2005 |
Cross sectional | May 2002-July 2003 | Tiljala, Kolkata | 263 | 2–5 | 53 | -- | -- | Direct wet preparation, saline and iodine mount, formalin concentration technique |
| Andhra Pradesh | ||||||||||
| 30 | Naish [40] 2004 |
Cross sectional | Dec. 1997-Dec.1998 | Peda Jalaripet Vishakhapatnam |
204 | 5–9 | 91 | 72 | 54 | Modified formol ether sedimentation technique was used |
| 31 | Padmaja [41] 2014 |
Cross sectional | Nov. 2013-January 2014 | Amalapuram | 200 | Around 8 | 6 | 7.5 | 4.5 | Saline and iodine preparations, formal ether concentration methods. |
| 32 | Panda [42] 2012 |
Cross sectional | Dec 2008-Jan 2009 | Kondavelagada village, Vizianagaram district | 124 | 6–9 | -- | -- | 4.8 | Saline& Iodine preparation |
| Puducherry | ||||||||||
| 33 | Ragunathan [43] 2010 |
Cross sectional | March-Sept. 2006 | Puducherry | 1172 | 5–10 | 43.21 | 10.87 | 28.89 | Formol ether sedimentation, iodine saline wet mount |
| Karnataka | ||||||||||
| 34 | Golia [44] 2014 |
Cross sectional | Jun-Sep 2013 | Kavalbyrasandra, Bangalore | 258 | 6–12 | 8.1 | 5.4 | 1.2 | Normal saline, iodine preparation and formalin ether sedimentation concentration technique. |
| Tamil Nadu | ||||||||||
| 35 | Kattula [45] 2014 |
Cross sectional | Dec. 2008-Aug-2009 | Vellore, thiruvanamalai | 3706 | 6–14 | 1.21 | 0.8 | 6.28 | Saline and iodine wet preparation, McMaster egg counting technique |
| 36 | Krishnan [46] 2013 |
Cross sectional | ND | Melmaruvathur | 25 | 5–10 | 20 | 5 | 10 | Saline and iodine preparation, salt flotation technique and zinc sulphate flotation technique |
| 37 | Fernandez [47] 2002 |
Cross sectional | Sept-March 2000 | Chennai Kadallur |
125 | 3–14 | 52.8 | 45.6 | 37.6 | Saline and Lugol’s iodine zinc sulphate concentration technique |
| 38 | Dhanabal [48] 2014 |
Cross sectional | Jan.-Jun 2013 | South Chennai | 256 | 0–50 | 6.2 | 1.1 | Saline and iodine wet mount, 10% formalin sedimentation and flotation | |
| 39 | Sunish [49] 2015 |
Cross sectional | 2001 | Villupuram (Tirukoilur) (Mugaiyur) |
321-T 325-M |
9–10 | 54.83-T 52.92-M |
4.67-T 6.77-M |
16.51-T 8.31-M |
Kato Katz cellophane thick smear technique |
(AL A. Lumbricoides, TT T. trichiura, HW Hookworm, ND Not defined)
More than 50% prevalence for T. trichiura was reported from two different locations from the states of Assam and Andhra Pradesh and more than 50% prevalence for hookworm was reported from a single location from the state of Andhra Pradesh. Open defecation practices, lack of personal and community sanitation, lack of footwear wearing habit, poor maternal education, low literacy rate and poor socio-economic status were significant predictors of prevalence of STH infections. Jalaripet in Andhra Pradesh was a unique location from where two reports were published 7 years apart [40, 50]. A weighted average indicated more than 50% prevalence for all three parasites (91.12% for A. lumbricoides, 71.5% for T. trichiura and 50.2% for hookworm). This was the only location from where such a high prevalence was reported of all three parasites by two studies despite the time gap. The states of Uttarakhand, Uttar Pradesh, Jharkhand, Manipur, Maharashtra and Puducherry reported a prevalence higher than 20%. Less than 20% prevalence was reported from another seven states of Delhi, Himachal Pradesh, Chandigarh, Madhya Pradesh, Rajasthan, Gujarat and Karnataka
Discussion
Soil-transmitted helminth infections continue to plague large parts of the world with India a significant contributor to the burden of disease [2]. Despite efforts to introduce usage of pit-latrines instead of open defecation, mass deworming program and improvement in water quality and sanitation, STH infections are still prevalent. A conducive climate for its growth, rapid and unplanned urbanization, social practices of open defecation and lack of community education and sanitation are some of the factors, which impedes control of infection in India. India undertook two massive deworming programme, one starting in year 2000 where a single dose of Albendazole and DEC was administered to filarial endemic regions and another in year 2015 covering 241 million children for treatment of STH infections. Although several studies have been published from different regions of India on this topic with the earliest scientific literature dating back as far as 1923 [51], the data on STH infections remains scattered. This information has the potential to inform and develop a comprehensive approach to control STH infections and target highly endemic areas with greater urgency.
The present study is a detailed effort to assess the burden of STH infections by searching past and present published literature and analyzing the prevalence of roundworm, whipworm and hookworm in one of the most endemic country in the world. The study covers 15 years of published literature on the topic of STH covering 19 out of the 29 Indian states. This study is another step in the direction of understanding prevalence and geographical distribution of STH diseases that affects nearly a one-sixth of world’s population living in India.
The most important factors affecting the survival and spread of STH infections are: the climate conditions, sanitation and socio-economic status. India has a range of climatic conditions, however except the arid and semi-arid region of Rajasthan other parts of India have largely tropical climate with high humidity and warm temperatures. Theses climatic conditions provide ideal environment for the survival of parasite eggs in moist soils, incidentally highest prevalence of STH infections was reported from such region of Tamil Nadu, Andhra Pradesh, Bihar, Assam, and West Bengal. A notable exception was Kashmir valley and Himachal Pradesh, which sees very cold and warm climates alternately, however, random urbanization without proper development of civic structure facilitates the spread of STH infections even in colder climates. Nearly 85% studies reported prevalence of STH infections in children, which form the disproportionately affected strata of society. Nearly 90% of the studies reported the prevalence of more than one parasite in same community, possibly because all the three helminths share similar climatic and socio-economic niche. Several studies reported cases of co-infection, with other protozoal parasites. Southern, northern and eastern parts of India are the most affected regions with A. lumbricoides the commonest parasite reported. WHO recommends annual deworming for areas with higher that 20% prevalence and biannual deworming for areas with higher than 50% prevalence (http://www.who.int/intestinal_worms/strategy/en/). Our study identifies six states with more than 20% prevalence and another six states with more than 50% prevalence rate, and in urgent need of chemotherapy. Very high prevalence was reported in Jalaripet in Andhra Pradesh implying that agriculture or farming based population may be at a greater risk due to higher exposure to contaminated soil and water at a regular basis [52, 53]. Many of these states (Delhi, Rajasthan, Tamilnadu, Assam) from which prevalence data is available, are tourist destinations. Travel based spread of vector borne diseases is well documented [54]. Similarly an exposure of naïve individuals to STH infections carries the risk of introducing STH to new locations, although tourist are a population that does not usually share lack of sanitation and poor socio-economic status and they are much less at risk of STH transmission.
Data extraction and compilation are prone to bias, to this effect we have made every effort to identify and screen published literature with broad search queries, nonetheless many relevant studies were inaccessible due to lack of full text availability. We excluded all the studies that were reported in clinical patients as STH infections are largely asymptomatic and community based cross sectional studies are better in assessing the burden of infection. Furthermore, all the studies with incomplete information were excluded which emphasizes the systematic collection of data in cross sectional studies. Additionally there was lack of publications from central, north western and southwestern region of India, with maximum studies being published from states of Tamil Nadu, Maharashtra, Assam, Uttar Pradesh and Jammu Kashmir. Odisha, Chhattisgarh and newly formed state of Telangana contribute a large section of people living below poverty line, however prevalence of this region could not be determined due to absence of any published reports. Also the review relied completely on published literature where grey literature and studies with minimal or negative results may not have been included resulting in publication bias. Another important factor that might lead to under reporting of STH infection is lack of surveillance data since STH infections are asymptomatic in nature. Only those having symptoms are expected to seek clinical intervention. A cross sectional survey from every state or union territory will accurately predict the prevalence and burden of STH infections.
WHO has recommended Kato-Katz method as the best and most reliable diagnostic tool with better efficacy, accuracy and predictive value than other techniques in resource poor settings [55]. However, only 15% studies reported the use of this method. Several states report less than 20% prevalence of STH infections. Kato-Katz technique has been shown to have reduced sensitivity in low transmission settings, which can be improved by taking more than one sample. Most of the studies relied on single stool examination, which may result in underreporting of the prevalence. Determination of prevalence and intensity of STH infection is an important tool for preventive chemotherapy and to assess the effect of ongoing deworming programs. Most of the studies have relied upon direct smear or in combination with flotation methods to determine the presence of helminth eggs. Adoption of Kato-Katz techniques with multiple stool samples will be helpful in identifying the actual prevalence of STH infections.
Despite these limitations, we were able to identify prevalence of STH infections in 19 states of India that covers nearly 84% of India’s population, clearly identifying regions of high prevalence which requires focused efforts of mass deworming to reduce parasitic load. A concomitant improvement in sanitation level will be helpful in reducing cases of reinfection. An operational focus on children by utilizing school infrastructure is generally the best approach. However targeting and educating mothers about the problems associated with helminth infections and open defecation must be emphasized by community-based programs. Systematic collection of survey reports based on WHO recommended diagnostic methods for parasitological testing should be undertaken. Such efforts will be helpful in identifying regions of high endemicity and monitor ongoing deworming programs. Geospatial mapping based on soil characteristic have been very useful in determining STH burden in other parts of the world. Such an approach could be valuable when survey data based on stool test are not available. Lastly, an overall improvement of sanitation and behavioral changes in the attitude of people will go a long way in avoiding reinfection and interrupting transmission of STH infections.
Conclusion
The present study attempts to compile available data about the prevalence of STH infections in India. This analysis is based upon the reporting of STH prevalence data in cross sectional studies carried out in different parts of India. We have also georeferenced affected regions to identify populations in need of annual or biannual deworming to control STH infections. This study will hopefully provide a guide map for the control of STH infections by preventive chemotherapy. Furthermore, lack of studies from several parts of India requires urgent attention for the surveillance and prevalence determination of STH infection. An exhaustive knowledge of the burden of disease will be helpful in allocating resources, funding and designing survey strategies for the control and monitoring of STH infections in Indian subcontinent.
Acknowledgements
N/A.
Funding
No funding was received for this work.
Availability of data and materials
The datasets analysed during the current study is available from the corresponding author on reasonable request.
Authors’ contributions
NS and SA searched the database for relevant publications. NS and SA extracted and analyzed the data from relevant publication. NS wrote the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
N/A.
Ethics approval and consent to participate
N/A.
References
- 1.Savioli L, Albonico M. Soil-transmitted helminthiasis. Nat Rev Microbiol. 2004;2:618–619. doi: 10.1038/nrmicro962. [DOI] [PubMed] [Google Scholar]
- 2.Lobo DA, Velayudhan R, Chatterjee P, Kohli H, Hotez PJ. The neglected tropical diseases of India and South Asia: review of their prevalence, distribution, and control or elimination. PLoS Negl Trop Dis. 2011;5:e1222. doi: 10.1371/journal.pntd.0001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.http://www.who.int/neglected_diseases/preventive_chemotherapy/sth/db/?units=minimal®ion=all&country=all&countries=all&year=all Accessed on 31 Oct 2015.
- 4.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 5.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001620. doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levecke B, Montresor A, Albonico M, Ame SM, Behnke JM, et al. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2014;8:e3204. doi: 10.1371/journal.pntd.0003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisson S, Sosai P, Ray S, Routray P, Torondel B, et al. Promoting latrine construction and use in rural villages practicing open defecation: process evaluation in connection with a randomised controlled trial in Orissa, India. BMC Res Notes. 2014;7:486. doi: 10.1186/1756-0500-7-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegelbauer K, Speich B, Mausezahl D, Bos R, Keiser J, et al. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia TW, Melville S, Utzinger J, King CH, Zhou XN. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wani SA, Ahmad F, Zargar SA, Dar PA, Dar ZA, et al. Intestinal helminths in a population of children from the Kashmir valley, India. J Helminthol. 2008;82:313–317. doi: 10.1017/S0022149X08019792. [DOI] [PubMed] [Google Scholar]
- 12.Wani SA, Ahmad F, Zargar SA, Amin A, Dar ZA, et al. Intestinal helminthiasis in children of gurez valley of Jammu and Kashmir state, India. J Glob Infect Dis. 2010;2:91–94. doi: 10.4103/0974-777X.62872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wani SA, Ahmad F, Zargar SA, Fomda BA, Ahmad Z, et al. Helminthic infestation in children of Kupwara district: a prospective study. Indian J Med Microbiol. 2007;25:398–400. doi: 10.4103/0255-0857.37348. [DOI] [PubMed] [Google Scholar]
- 14.Singh C, Zargar SA, Masoodi I, Shoukat A, Ahmad B. Predictors of intestinal parasitosis in school children of Kashmir: a prospective study. Trop Gastroenterol. 2010;31:105–107. [PubMed] [Google Scholar]
- 15.Ranjan S, Passi SJ, Singh SN. Prevalence and risk factors associated with the presence of Soil-Transmitted Helminths in children studying in Municipal Corporation of Delhi Schools of Delhi, India. J Parasitic Dis. 2013;39:377–384. doi: 10.1007/s12639-013-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehgal R, Reddy GV, Verweij JJ, Rao AVS. Prevalence of intestinal parasitic infections among school children and pregnant women in a low socio-economic area, Chandigarh, North India. RIF. 2010;1:100–103. [Google Scholar]
- 17.Bora D, Meena VR, Bhagat H, Dhariwal AC, Lal S. Soil transmitted helminthes prevalence in school children of Pauri Garhwal District, Uttaranchal state. J Commun Dis. 2006;38:112–114. [PubMed] [Google Scholar]
- 18.Barda B, Ianniello D, Salvo F, Sadutshang T, Rinaldi L, et al. “Freezing” parasites in pre-Himalayan region, Himachal Pradesh: Experience with mini-FLOTAC. Acta Trop. 2014;130:11–16. doi: 10.1016/j.actatropica.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Bisht D, Verma AK, Bharadwaj HH. Intestinal parasitic infestation among children in a semi-urban Indian population. Trop Parasitol. 2011;1:104–107. doi: 10.4103/2229-5070.86946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid M, Joshi M, Joshi H, Fatemi K. Prevalence of intestinal parasites among school going children in Bareilly District. 2011. [Google Scholar]
- 21.Nitin S, Venkatesh V, Husain N, Masood J, Agarwal GG. Overview of intestinal parasitic prevalence in rural and urban population in Lucknow, north India. J Commun Dis. 2007;39:217–223. [PubMed] [Google Scholar]
- 22.Awasthi S, Verma T, Kotecha PV, Venkatesh V, Joshi V, et al. Prevalence and risk factors associated with worm infestation in pre-school children (6-23 months) in selected blocks of Uttar Pradesh and Jharkhand, India. Indian J Med Sci. 2008;62:484–491. doi: 10.4103/0019-5359.48552. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi K, Nema S, Bankwar V, Dhanvijay AK. Intestinal Parasitic infections and Demographic status of school children in Bhopal region of Central India. J Pharm Biol Sci. 2014;9:83–87. [Google Scholar]
- 24.Choubisa SL, Jaroli VJ, Choubisa P, Mogra N. Intestinal parasitic infection in Bhil tribe of Rajasthan, India. J Parasitic Dis. 2012;36:143–148. doi: 10.1007/s12639-012-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shobha M, Bithika D, Bhavesh S. The prevalence of intestinal parasitic infections in the urban slums of a city in Western India. J Infect Public Health. 2013;6:142–149. doi: 10.1016/j.jiph.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Lakhani JS, Rana R, Joshi S, Vasisht S. Intestinal parasitic infestations among school children in Piparia Village, Vadodara District. Int J Sci Res. 2013;2:434. [Google Scholar]
- 27.Pandey B, Kumar D, Verma D. Epidemiological study of parasitic infestations in rural women of Terai belt of Bihar, India. Ann Biol Res. 2013;4:30–33. [Google Scholar]
- 28.Greenland K, Dixon R, Khan SA, Gunawardena K, Kihara JH, et al. The epidemiology of soil-transmitted helminths in Bihar State, India. PLoS Negl Trop Dis. 2015;9:e0003790. doi: 10.1371/journal.pntd.0003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narain K, Medhi GK, Rajguru SK, Mahanta J. Cure and reinfection patterns of geohelminthic infections after treatment in communities inhabiting the tropical rainforest of Assam, India. Southeast Asian J Trop Med Public Health. 2004;35:512–517. [PubMed] [Google Scholar]
- 30.Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RCA. The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Health. 2004;9:688–701. doi: 10.1111/j.1365-3156.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 31.These ME. Burden of Ascariasis in schoolchildren of Assam. J Commun Dis. 2009;41:289–292. [Google Scholar]
- 32.Sharma SK, Narain K, Devi KR, Mohapatra PK, Phukan RK, et al. Haemoglobinopathies–major associating determinants in prevalence of anaemia among adolescent girl students of Assam, India. 2012. [DOI] [PubMed] [Google Scholar]
- 33.Singh HL, Singh NB, Singh YI. Helminthic infestation of the primary school-going children in Manipur. J Commun Dis. 2004;36:111–116. [PubMed] [Google Scholar]
- 34.Pukhrambam R, Ranjan RK, Chaudhuri S, Mukhia S. Prevalence and risk factors of soil -transmitted helminth infections among the under-five children in an urban slum of Manipur New Indian. J Pediatr. 2013;2:62–68. [Google Scholar]
- 35.Dambhare D, Bharambe M, Garg B. Intestinal parasites prevalence and related factors among school children in the rural area of central India. J Commun Dis. 2010;42:281–286. [PubMed] [Google Scholar]
- 36.Aher A, Kulkarni S. Prevalence of intestinal parasites in school going children in a rural community. Int J Biomed Res. 2012;2:605–607. doi: 10.7439/ijbr.v2i12.205. [DOI] [Google Scholar]
- 37.Avhad S, Hiware C. Soil transmitted helminthiasis among school age children in Aurangabad District, Maharashtra State, India. Prevalence. 2012;10:100. [Google Scholar]
- 38.Mukherjee AK, Chowdhury P, Das K, Raj D, Karmakar S, et al. Helminth burden among school going children of southern Bengal, India: A survey report. Glob J Biol Agri Heal Sci. 2013;2(3):189–91.
- 39.Sur D, Saha DR, Manna B, Rajendran K, Bhattacharya SK. Periodic deworming with albendazole and its impact on growth status and diarrhoeal incidence among children in an urban slum of India. Trans R Soc Trop Med Hyg. 2005;99:261–267. doi: 10.1016/j.trstmh.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Naish S, McCarthy J, Williams GM. Prevalence, intensity and risk factors for soil-transmitted helminth infection in a South Indian fishing village. Acta Trop. 2004;91:177–187. doi: 10.1016/j.actatropica.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Padmaja N, Swaroop PS, Nageswararao P. Prevalence of Intestinal Parasitic Infections among School Children in and around Amalapuram. J Pub Health Med Res. 2014;2(2):36-8.
- 42.Panda S, Rao UD, Sankaram KR. Prevalence of intestinal parasitic infections among school children in rural area of Vizianagaram. IOSR J Pharm Biol Sci. 2012;3:42–45. [Google Scholar]
- 43.Ragunathan L, Kalivaradhan SK, Ramadass S, Nagaraj M, Ramesh K. Helminthic infections in school children in Puducherry, South India. J Microbiol Immunol Infect. 2010;43:228–232. doi: 10.1016/S1684-1182(10)60036-9. [DOI] [PubMed] [Google Scholar]
- 44.Golia S, Sangeetha K, Vasudha C. Prevalence of parasitic infections among primary school children in bangalore. Int J Basic Appl Med Sci. 2012;4:12–18. [Google Scholar]
- 45.Kattula D, Sarkar R, Ajjampur SSR, Minz S, Levecke B, et al. Prevalence & risk factors for soil transmitted helminth infection among school children in south India. Indian J Med Res. 2014;139:76–82. [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnan A, Sekar U, Sathanantham DK. Prevalence and Pattern of Helminthic Infection among Children in a Primary School of Rural Tamil Nadu. Acad Med J India. 2013;1:40–2.
- 47.Fernandez MC, Verghese S, Bhuvaneswari R, Elizabeth SJ, Mathew T, et al. A comparative study of the intestinal parasites prevalent among children living in rural and urban settings in and around Chennai. J Commun Dis. 2002;34:35–39. [PubMed] [Google Scholar]
- 48.Dhanabal J, Selvadoss PP, Muthuswamy K. Comparative study of the prevalence of intestinal parasites in low socioeconomic areas from South chennai, India. J Parasitol Res. 2014;2014:630968. doi: 10.1155/2014/630968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunish I, Rajendran R, Munirathinam A, Kalimuthu M, Kumar VA, et al. Impact on prevalence of intestinal helminth infection in school children administered with seven annual rounds of diethyl carbamazine (DEC) with albendazole. Indian J Med Res. 2015;141:330. doi: 10.4103/0971-5916.156622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mani GG, Rao ST, Madhavi R. Estimation of hookworm intensity by anthelmintic expulsion in primary schoolchildren in south-india. Trans R Soc Trop Med Hyg. 1993;87:634–635. doi: 10.1016/0035-9203(93)90268-U. [DOI] [PubMed] [Google Scholar]
- 51.Hookworm disease in India. Br Med J. 1923;76.
- 52.Clark A, Turner T, Dorothy KP, Goutham J, Kalavati C, et al. Health hazards due to pollution of waters along the coast of Visakhapatnam, east coast of India. Ecotoxicol Environ Saf. 2003;56:390–397. doi: 10.1016/S0147-6513(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 53.Ensink JH, Blumenthal UJ, Brooker S. Wastewater quality and the risk of intestinal nematode infection in sewage farming families in hyderabad, India. Am J Trop Med Hyg. 2008;79:561–7. http://apps.webofknowledge.com/full_record.do?product=WOS&search_mode=GeneralSearch&qid=2&SID=4Ews1NjaqvMUbdx4wqN&page=1&doc=1. [PMC free article] [PubMed]
- 54.Nunes MR, Palacios G, Faria NR, Sousa EC, Jr, Pantoja JA, et al. Air travel is associated with intracontinental spread of dengue virus serotypes 1-3 in Brazil. PLoS Negl Trop Dis. 2014;8:e2769. doi: 10.1371/journal.pntd.0002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolay B, Brooker SJ, Pullan RL. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. Int J Parasitol. 2014;44:765–774. doi: 10.1016/j.ijpara.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study is available from the corresponding author on reasonable request.


