Abstract
Several dermatoses are routinely associated with diabetes mellitus, especially in patients with chronic disease. This relationship can be easily proven in some skin disorders, but it is not so clear in others. Dermatoses such necrobiosis lipoidica, granuloma annulare, acanthosis nigricans and others are discussed in this text, with an emphasis on proven link with the diabetes or not, disease identification and treatment strategy used to control those dermatoses and diabetes.
Keywords: Dermatology, Diabetes mellitus, Skin manifestations
INTRODUCTION
Diabetes mellitus (DM) is considered a modern epidemic disease that affects about 8.3% of adults, which accounts for 382 million people of the global population, and 46% of cases are estimated to be currently undiagnosed.1
The increasing urbanization with dietary changes, reduced physical activity, and changes in other lifestyle patterns, in addition to the increasing rates of obesity contributes to the greater prevalence of DM.
Besides the severe renal, vascular and ophthalmic complications, the skin may be compromised by various diseases directly related to diabetes or with associations not yet fully proven. The main ones are discussed in this text, with an emphasis on proven link with diabetes or not, disease identification and treatment strategy used to control these dermatoses and diabetes.
ACANTHOSIS NIGRICANS
Acanthosis nigricans (AN) is characterized by skin thickening with hyperchromic and a velvety aspect that occurs mainly in the folds, especially in the armpits (Figure 1). Currently, it is postulated that is caused by hyperinsulinemia, which promotes the synthesis of type 1 insulin growth factor (IGF1) that leads to epidermal acanthosis.2 Furthermore, AN can be associated to skin tags (achrocordons) and acral papilosis, which can help in screening insulin resistance among general population.
Figure 1.
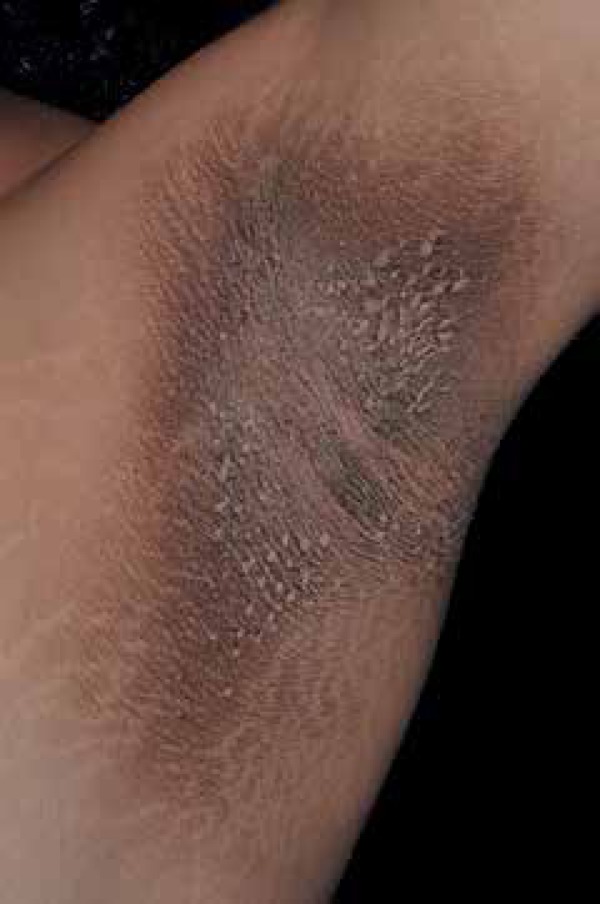
Armpits are a classic location of acanthosis nigricans. Note the thickening and hyperchromia of the skin
Photo: Department of Dermatology, Botucatu Medical School, UNESP
The disease may also be associated with certain malignancies such as gastric cancer and high doses of niacinamide, but in most cases the patient has also type A insulin resistance, although adrenal and thyroid disease may be associated.3-5
In obese patients, AN seems to evolve in association with the metabolic syndrome. The most affected sites are the armpits, neck, areolas, umbilicus and elbows. Topical treatment using emollients with basis of urea and oral metformin can be used (due to insulin resistance), but the only effective measures are weight loss and physical exercises, which reverses the metabolic disturbances that causes cutaneous manifestations.
BULLOSIS DIABETICORUM
Bullosis diabeticorum (BD), bullous disease of diabetes or diabetic blisters occurs in approximately 0.5% of diabetic patients.6 It was first described in 1930, but only in 1967 the term bullosis diabeticorum was proposed.7,8 Even though uncommon, it can be considered a distinct marker of DM and it is manifested in patients with long history of evolution of diabetes or those who have complications such as nephropathy or neuropathy, although there are reports of concomitant appearance to the initial presentation of DM.8
BD has been reported in patients aged 17 to 80 years with a larger proportion in males (2:1). The preferred locations are the extremities, especially legs and feet (Figure 2).9
Figure 2.
Bullosis diabeticorum blisters are asymptomatic and exhibit mild inflammation
Photo: Department of Dermatology, Botucatu Medical School, UNESP
Pathophysiology of the BD bullae is still unknown. The blisters are large, tense and characterized by sudden and spontaneous onset in acral regions.9,10 The diameter of the blisters varies between 0.5 and 5cm, they are often bilateral, with an inflammatory base, and contain a clear, sterile, nonserous content.10 Other affected places are the back and side of the hands and the arms.10 These blisters are usually painless and non-pruritic and disappear spontaneously without scarring in 2 to 5 weeks.10,11
Outbreaks may occur, but risk factors are radiation and trauma, blood glucose changes, magnesium and calcium alterations, vascular disease or microangiopathy and kidney failure.9,11-15
Diagnosis is made on clinical basis and should be remembered when there are large blisters without apparent inflammation in longstanding diabetic patients or those with chronic complications of the disease.
Histologically, the blisters are manifested in three different types based on the level of cleavage. The most common type shows an subepidermal cleavage at the level of the lamina lucida without acantholysis, which appear and disappear spontaneously, without scarring.8,14 Blisters present hyaline content and are located at the tips of the toes and less frequently in the dorsal surfaces of the feet. Patients with these clinical manifestations have good circulation in the affected limb and tend to present diabetic peripheral neuropathy.
The second type is rarer and involves lesions that may be hemorrhagic including resolution with scars and atrophy.16 The cleavage plane is below the dermoepidermal junction, with destruction of anchoring fibrils.17,18 A third described type consists of multiple blisters associated with sun exposure and markedly tanned skin. It affects feet, legs and arms and must be distinguished from porphyria cutanea tarda.18
The differential diagnosis includes pemphigus, bullous pemphigoid, contact dermatitis, insect bites, epidermolysis bullosa, blisters by trauma, burns, bullous erysipelas, bullous drug eruptions and porphyria cutanea tarda.8,9,19
DIABETIC DERMOPATHY
Diabetic dermopathy (DD) is the most common specific skin lesion in patients with diabetes. 20,21 The disease was first described by Hans Melin in the early 60s, as circumscribed brownish lesions located in the lower limbs of diabetic patients and named as diabetic dermopathy by Binkley (1965), who considered it a cutaneous manifestation of diabetic microangiopathy. 22,23
Its incidence may range from 7% to 70% of diabetic patients.5,20,22,25 DD is seen more often in older patients, aged more than 50 years, and in those with a long history of diabetes.18,20 Also, it is more common in men (2: 1).20,22,26,27 There is some controversy as to DD be a pathognomonic sign for diabetes since there are studies that have shown its involvement in non-diabetic subjects.6,27
The origin of DD is unknown and there is no relation with decreased local perfusion.28 Another possible explanation is due to mild traumas that do not compromise wound healing.29,30 There is also degeneration of subcutaneous nerves in patients with neuropathy.31 However, the most acceptable explanation is the relation between DD and microvascular complications of diabetes. Studies have shown strong association with DD, nephropathy, retinopathy or neuropathy.20,30
Shemer et al.20 observed increased incidence of DD in 52-81% when associated with such complications. Another study showed that 42.9% of patients presented neuropathy associated with DD (p<0.01)6 although about 21% of patients with DD showed no evidence of microangiopathy. 20
The association between DD and cardiovascular disease has also been identified based on ECG changes, history of coronary artery disease or both. About 53% of patients with type 2 diabetes and DD had coronary artery disease.6 DD association with neuropathy, nephropathy, retinopathy and coronary artery disease may indicate a severity marker of the evolution of diabetes.31
As DD tends to occur over bony prominences, it is suggested that occur in response to sudden trauma.11,14,20,31 The association between trauma and DD lesions is further confused by the frequent presence of peripheral neuropathy.31 Nevertheless, some studies have failed to induce DD in vivo.32
DD consists of small, well-defined surface, brownish depressions, with atrophic appearance, resembling scars. Commonly the lesions measure less than 1cm in diameter and present rounded shape (Figure 3). They can occasionally extend and reach up to 2.5cm. Depressions are smooth and hyperpigmented and intensity of the pigment is related to the degree of atrophy. Generally asymptomatic, it does not cause pain or itching and is typically located bilaterally in pretibial regions and distributed asymmetrically.21 More rarely, DD occurs on the thighs, trunk and lower abdomen.6,21,22 The location and atrophic appearance causes many patients to consider DD as scars resulting from a possible trauma.21,22 The appearance of DD at the beginning is hardly documented, being an underreported disease.
Figure 3.
Diabetic dermopathy consists of small brownish-colored depressions in the skin surface, of atrophic appearance, which look like scars
Photo: Department of Dermatology, Botucatu Medical School, UNESP
The progression of DD is variable and does not appear to be affected by glycemic control.5,24,25 Individual lesions may persist on average for 18-24 months and may stay indefinitely. When the disease regresses, the process is slow can be solved completely or maintain pigmentation without atrophy. Cyclically, older lesions disappear and new ones continuously evolve.21,22,23,32
The diagnostic is clinically based: after careful history and physical examination, diagnosis of DD becomes evident. The presence of multiple, hyperpigmented, sharply demarcated atrophic scars in the lower leg of a patient with diabetes is highly suggestive of DD. The presence of four or more typical lesions in diabetic patients is also characteristic of DD.10 Biopsy is not routinely performed, since the histology is not specific and it is interesting avoid trauma to the lower extremities in these individuals. However, atypical features or unusual locations may hinder the diagnosis and recommend the histopathological examination.31,32
Histologic findings include atrophy of the dermal papillae, variable pigment at basal cells, thickening of the superficial blood vessels intima, hypertrophy and hyalinization of the deepest arterioles, extravasated erythrocytes, hemosiderin deposition and a mild lymphocytic infiltration.19,21,24 There is telangiectasia, edema, and fibroblast proliferation at the papillary dermis.
The differential diagnosis of DD includes many diseases. Early lesions of DD can be mistaken with fungal infection.23, While typical brownish atrophic scars may require differentiation of Schamberg’s disease (progressive pigmented purpuric dermatitis), purpura annularis telangiectasica, purpuric lichenoid dermatitis, pigmented stasis dermatitis, scarring lesions, papulonecrotic tuberculids, factitious dermatitis and abrasions.23 Many of these entities can be differentiated by distribution, appearance and natural history.
Treatment of DD is not recommended and is little effective.30 Lesions are asymptomatic and can persist indefinitely or make spontaneous regression without treatment.21 Nevertheless, the conditions associated with DD require attention. Patients should be evaluated for the diagnosis of DM, which when is not confirmed, should require further investigations. Once confirmed the presence of diabetes, attention should be focused on prevention, detection and control of associated complications. As with all patients with diabetes, glycemic control is critical.
SCLERODERMIFORM DISORDERS
Patients with diabetes may have thickening and hardening of the skin of the dorsal region of the finger as well as the skin overlying the joints of the hand and fingers. The sclerosis can even extend these places. These changes are more common in type 1 diabetes and occur in up to 50% of the patients. The cause seems to be the glicosylation of proteins that appears to cause hardening of the skin.
Another form of skin sclerosis is associated with diabetes is the scleredema adultorum of Buschke (SAB), where sclerosis is diffuse, but located preferably on the back, having an erythematous appearance, and which may compromise the neck, shoulders, and even other regions. It is more common in men over 40 years with insulindependent or multiple complications.
SAB is a rare fibromucinous connective tissue disease of unknown etiology resistant to therapy and without spontaneous resolution.33-35 It is characterized by symmetrical and diffuse thickening with hardening of the skin affecting mainly the face, trunk, neck and upper limbs, sparing the hands and feet (Figure 4).36,37 The disease presents no race, gender or age group preferences, however, it is more common in middle-aged men. DM is associated with about 50% of cases.38 Its prevalence varies between 2.5 and 14% in diabetic patients, but it is noteworthy that most of the cases are underdiagnosed. 35,39,40
Figure 4.
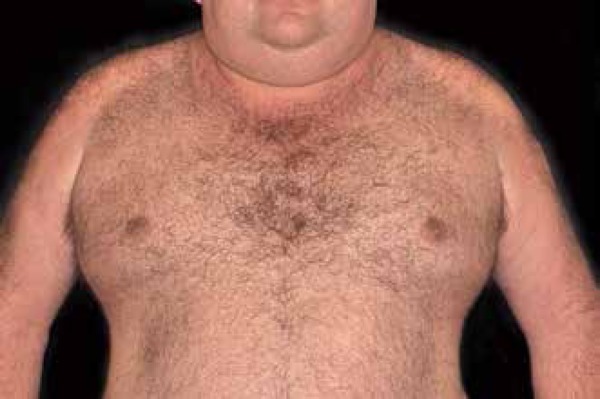
Buschke's scleredema is characterized by symmetrical anddiffuse thickening, with hardening of the skin mainly on the face, cervical region and upper limbs
Photo: Department of Dermatology, Botucatu Medical School, UNESP
SAB has an insidious, asymptomatic, onset with progressive loss of skin natural marks. In severe cases it can lead to neck and back pain.41 Mobility is reduced and may lead to a restrictive respiratory syndrome due to the skin thickening.42 The affected area is painless and can present decreased sensitivity to touch.41 Visceral involvement is rare, affecting eyes, tongue, pharynx, esophagus, musculoskeletal tissue, joints, heart (pericardial and pleural effusion) and hepatosplenomegaly.40,42,43
SAB belongs to the group of cutaneous mucinoses and can be associated with bacterial, viral, hematological disorders, diabetes and other endocrine disorders.37,44 Three scleredema variants are classically described.45
Type 1 - Occurs most often in middle-aged women, children and young people, presents acute onset and is associated with a febrile respiratory illness, most commonly streptococcal or viral (influenza, chicken pox, measles, cytomegalovirus and HIV).46,47 This variant has selfresolution after several months or years.
Type 2 - There is no relation with infections, it is slowly progressive and is associated with monoclonal gammopathy.48 This type tends to persist for years and may be at increased risk for multiple myeloma, being associated with other diseases such as amyloidosis, rheumatoid arthritis, Sjögren’s syndrome, obstructive sleep apnea, primary hyperparathyroidism, pituitary adenoma and adrenocortical disease. 40,44,49,50
Type 3 - associated to diabetes, which can be either of type 1 and type 2.33,51-54 It occurs generally in obese patients with long standing diabetes and poor metabolic control, microangiopathy and need for insulin.35,38,40 It affects middle-aged men with history of longtime DM. This type also tends to persist and there is no clear relation to prognosis or glycemic control.55
The diagnosis of SAB is clinical, but diagnostic imaging (e.g. ultrasonography and magnetic resonance) can help in assessing the extent or disease activity.56,57 Due to the lack of skin elasticity and the skin thickening in scleredema, incisional biopsy is usually recommended to confirm the diagnosis.36
Histopathology shows marked thickening of the reticular dermis (2 to 3 times) with caliber collagen bundles separated by bands of hyaline deposit mucin or hyaluronic acid best evidenced at toluidine blue staining. The glycosaminoglycan deposit histologically corresponds to an hyperintensity on magnetic resonance.58
Clinically, the differential diagnosis must be established with scleroderma, eosinophilic fasciitis and scleromyxedema.59
In SAB, the main mechanism of accumulation of extracellular matrix components appears to be represented by an abnormal gene expression of extracellular protein (collagen type 1, fibronectin, and type 3) on the skin instead of decreasing clearing processes. This deregulated gene is observed in SAB regardless of the presence of diabetes. The mediators of fibroblast activation are still unknown. 40 Although disappointing response to treatment, various therapeutic modalities are used: immunosuppressants (e.g. cyclosporine and methotrexate), pentoxifylline, prostaglandin E1, intravenous immunoglobulin, penicillamine, antibiotics, systemic corticosteroids, and intralesional, factor XIII, aminobenzoate, colchicine and DMSO gel, radiotherapy, photochemotherapy with psoralen and ultraviolet A (PUVA), and recently, tamoxifen and irradiation with electron beam.36,41,42,50,59,60 The therapy may be effective, probably due to the upregulation of collagenase synthesis by fibroblasts and subsequent degradation of collagen fibers.33,60,61
In general, the disease has a good prognosis, and in most cases is self-limited; however, there are severe cases with rapid progression, and it is hard to determine the best treatment for controlling the disease and analyzing the cost benefit ratio.
It is noteworthy that its chronicity can cause alterations in movement of the shoulders and impaired respiratory function.40
GRANULOMA ANNULARE
Granuloma annulare (GA) is a rare, benign and self-limited dermatitis of the pretibial regions and the extensor surfaces of the limbs. The cutaneous lesions are similar to necrobiosis lipoidica diabeticorum, but without causing atrophy of the epidermis.62,63 It is characterized by papules that often assume an annular configuration.63
Its etiology is unknown, but appears to be involved with response to infections such as HIV, hepatitis C, toxic agents, thyroid diseases and malignancy.64,65 GA association with diabetes is controversial and has been extensively studied. Samlaska et al. (1992), in a casecontrol study, revealed no statistically significant correlation between GA and type 2 DM, while in a retrospective study 12% of patients with GA had diabetes. Other studies have associated GA with DM also in about 12% of the patients.66,68
GA affects twice as many women than men and the most commonly affected areas are those exposed to trauma, such as backs of the hands and feet, fingers, elbow, arms and legs; sometimes the scalp may be affected (Figures 5 and 6).63 When GA is generalized, the trunk is affected in almost all cases. 64 In most cases the plaques are asymptomatic, but may present mild and occasional itching or a burning sensation.69
Figure 5.
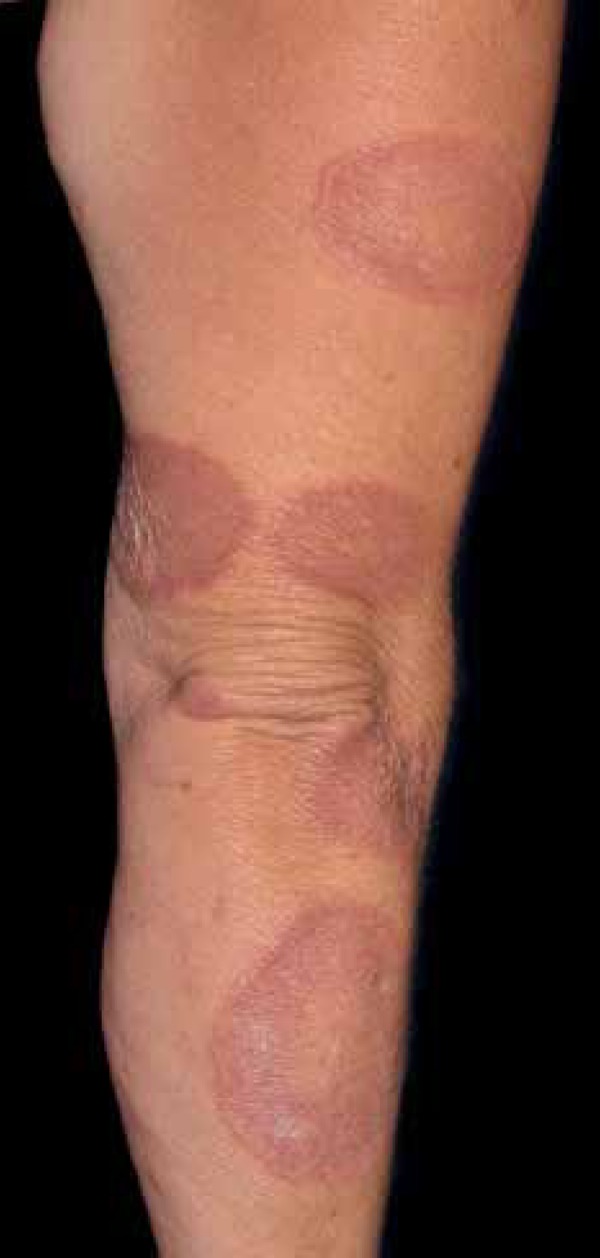
Granuloma annulare manifests by erythematous and firm dermal papules that expand gradually, with central hyperpigmentation
Photo: Department of Dermatology, Botucatu Medical School, UNESP
Figure 6.
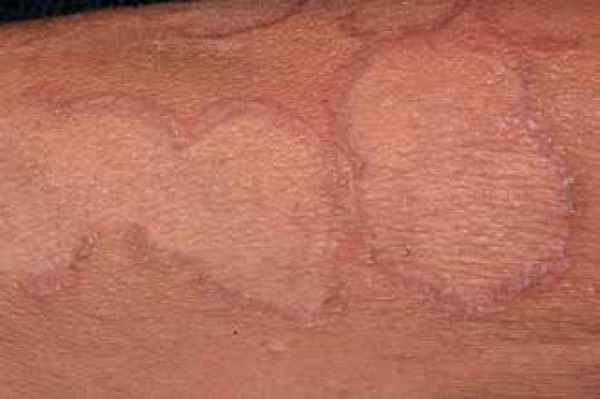
Detail of the granuloma annulare, showing infiltration at the edges of the lesion
Photo: Department of Dermatology, Botucatu Medical School, UNESP
The lesions begin as firm, skincolored dermal papules, which expand gradually in a centrifugal way. The format is annular, with a central hyperpigmentation, and sometimes the papules are frankly erythematous, becoming erythematous-brownish posteriorly.70 The papules of annular shape grow slowly and can measure from 0.5 to 5.0cm.63
GA affects mainly children and young people without diabetes but, in adults with diabetes, a disseminated form can occur, which is expressed in about 0.5% to 10% of these patients.63,64,66 The generalized perforating form is characterized by umbilicated papules of about 4mm located at the extremities and it is most commonly seen in children and young adults.
The probable pathophysiology is a stimulus that triggers the release of lymphokines by previously activated lymphocytes. These lymphokines stimulate the synthesis and activity of collagenase, producing an inflammatory reaction that modulates the formation of granulomas.63
The duration of the disease is highly variable. Many lesions disappear spontaneously, without scarring, but it can last for months to years. Disappeared lesions have about 40% chance to reappear.63 The lack of symptoms, scaling or blistering associated to GA helps to differentiate it from other skin diseases such as tinea corporis, pityriasis rosea, psoriasis, or annular erythema. Rarely, a biopsy is needed to confirm the diagnosis. 66
Histologically, GA appears as a focal degeneration of collagen in the upper and middle layers of the dermis, accumulation of histiocytes and multinucleated giant cells arranged in fence/palisade.14 Although histology is very similar to that observed in necrobiosis lipoidica, prominent mucin deposits in GA helps to differentiate it.
GA has a poor therapeutic response. Treatment usually is not necessary because most of its injuries remit spontaneously within two years.69 If the lesions become an unpleasant problem, the available options include high-dose topical steroids, intralesional injection of corticosteroids, PUVA, cryotherapy, or drugs such as niacinamide, infliximab, dapsone and topical calcineurin inhibitors. 69,70 Oral isotretinoin can be effective in symptomatic patients and the improvement of lesions occurs in 90% of those with decreased itching and erythema, even in resistant lesions associated with few adverse events compared with other drugs.11 Moreover, this treatment provides good aesthetic response with a considerable improvement in patient quality of life.
NECROBIOSIS LIPOIDICA DIABETICORUM
Necrobiosis lipoidica (NL) is an idiopathic dermatosis of unknown origin, occurring mainly in patients with diabetes. While most diabetics do not develop this disease, its incidence ranges from 0.3% to 1.6% of these patients per year.71
Two thirds of diabetics with NL are insulin dependent.72 NL is not exclusive to diabetics because up to a third of cases occur in non-diabetic subjects.73,74 Over the years, however, about 90% of these will develop some degree of glucose intolerance or at least will present a positive family history for diabetes.75,76,77 These facts suggest that as soon as the diagnosis of the dermatosis is confirmed, the research for diabetes should be initiated.
NL predominates in women (80% of cases), white, and it manifests at any age, but prevails between the fourth and sixth decades.75 A retrospective study from the Mayo Clinic showed that the confirmed diagnosis of diabetes, abnormal plasma glucose or a family history of diabetes occurred in 90% of patients.75
The glycated hemoglobin levels were not associated with the appearance of lesions, indicating that hyperglycemia is not necessary for the development of NL. Among yuastdua diabetes, type 1 patients have the earliest manifestations of NL.76
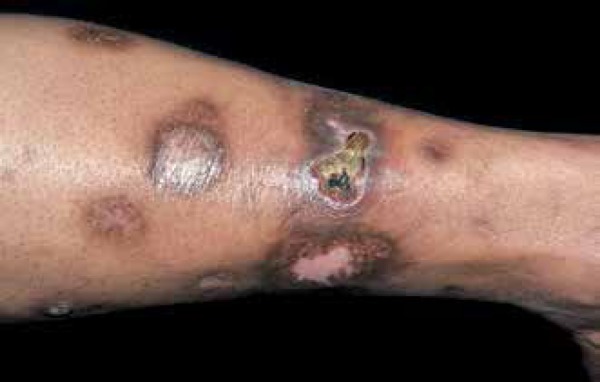
Multiple lesions are common, and are usually observed in both legs (Figures 7 and 8).77 Approximately 35% of the lesions progress to ulceration.78 Patients occasionally present itching or burning sensations in areas where they were asymptomatic and pain arises after ulceration. Some patients report partial or complete anesthesia at affected sites, due to probable local neural dysfunction.79 More than half of diabetic patients with NL have neuropathy or microangiopathy. Spontaneous resolution is observed in 10% to 20% of cases.
Figure 7.
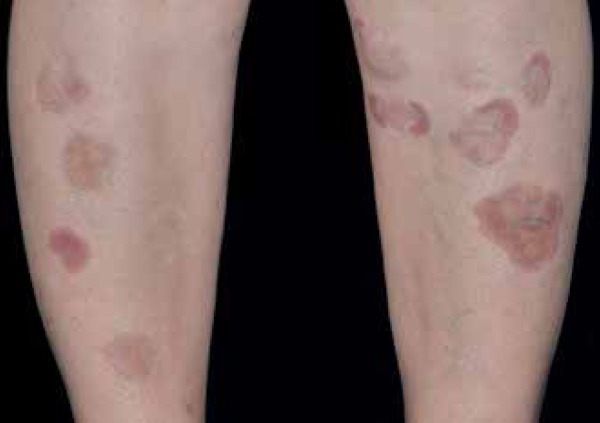
Typical lesions of necrobiosis lipoidica begin in the pretibial regions with non-squamous papules that gradually grow and group into large plaques
Photo: Department of Dermatology, Botucatu Medical School, UNESP
Figure 8.
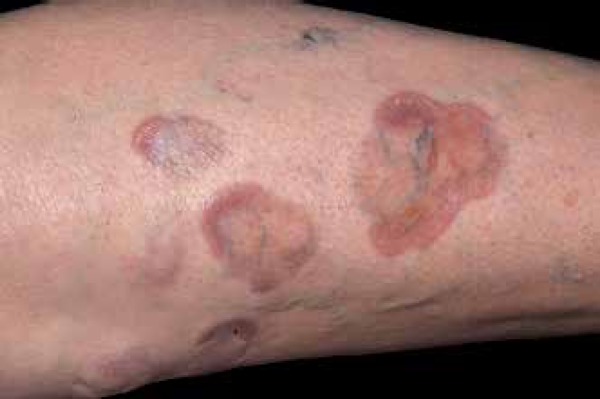
Detail of lesions of necrobiosis lipoidica, showing central atrophy
Photo: Department of Dermatology, Botucatu Medical School, UNESP
Histopathology shows disorganization and degeneration of collagen in basement membrane thickening and inflammation of the underlying subcutaneous fat. NL pathophysiology is still unclear. The primary cause of collagen degeneration appears to be an immunemediated vasculitis (autoimmune vasculitis). The presence of antibodies and C3 at the dermoepidermal junction and around the blood vessel vasculitis lesions support this, but other histological features of leukocytoclastic vasculitis were not observed. 80 Other studies suggest that NL is primarily a collagen disease with secondary inflammation, 81-83 and the presence of fibrin lesions associated with histiocytes in palisades suggests delayed hypersensitivity reaction.84
Typical lesions of NL start in the pretibial areas with nonscaly erythematous papules that gradually enlarge and coalesce into large plaques.85 The plaques result from the confluence of yellowish papules and often develop atrophic center that corresponds to the dermal and epidermal atrophy associated with superficial telangiectasias. 86
Gradual expansion and variable erythema occur at the edges, which are often elevated. Its shape is elliptical, with serpiginous margins. The adjacent skin is reddishviolet, while the center is yellow, indicating accumulation of lipids.77,78,87 The size of the lesion may range from a few millimeters to several centimeters. When the lesions become chronic, the sclerosis is well marked with porcelaneous aspect. The metabolic control appears to have no proven effect in the course of the disease, although there is a report that tight glucose control reduces the incidence of NL.88,89
The lesions may occasionally appear in other areas, such as the thighs, popliteal region and feet. Other sites are involved in 15% of cases and include abdomen, upper limbs (especially hands and forearms) and scalp, where NL can cause atrophy and alopecia. In the face, the disease may harm the eyelids and nose. In rare cases, lesions were observed in the heel and penis.89 NL also can develop in posttrauma scars, old lesions of scleroderma and at the scars of BCG vaccine.79 When lesions arise on other parts of the body, generally the lower limbs are affected too.
Diagnosis is made by clinical examination. Histopathological examination may be required in early lesions or in patients without a diagnosis of diabetes. Sarcoidosis, granuloma annulare, lichen sclerosus et atrophicus and stasis dermatitis may be differential diagnosis of NL. Ulcerated lesions can resemble pyoderma gangrenosum, tertiary syphilis and cutaneous mycobacteriosis.
Patients should be advised to avoid potentially traumatic situations, such as contact sports. They should the advised to wear socks up to the knee or foam pads for protection.79 In general, drug treatment has little effect and should be reserved for symptomatic relief. Drugs used with variable efficacy are intralesional injection and oral use of corticosteroids or topical threads under occlusion, clofazimine, acetylsalicylic acid, dipyridamole, pentoxyphiline and chloroquine.
When the lesions are flat, use of emollients is indicated. Rhodes (1980) reported success with fibrinolytic therapy (derived from nicotinic acid and inositol nicotinate) in 24 of 30 cases; redness and warmth are important adverse events.75
More recently, anti-TNF therapies have been used, although there are no studies that demonstrate decisive therapeutic efficacy yet.90 Strict glycemic control remains controversial in improving NL. When the plaques become ulcerated, the treatment must involve the prevention of secondary infection with systemic antibiotics and dressings. 82,91,92
DIABETIC FOOT
The diabetic foot is a complex and disabling entity, caused by various factors, and it should be treated by various specialties such as general surgery, vascular surgery, orthopedics, endocrinology and dermatology. It burdens patients’ quality of life, public health system and social security.
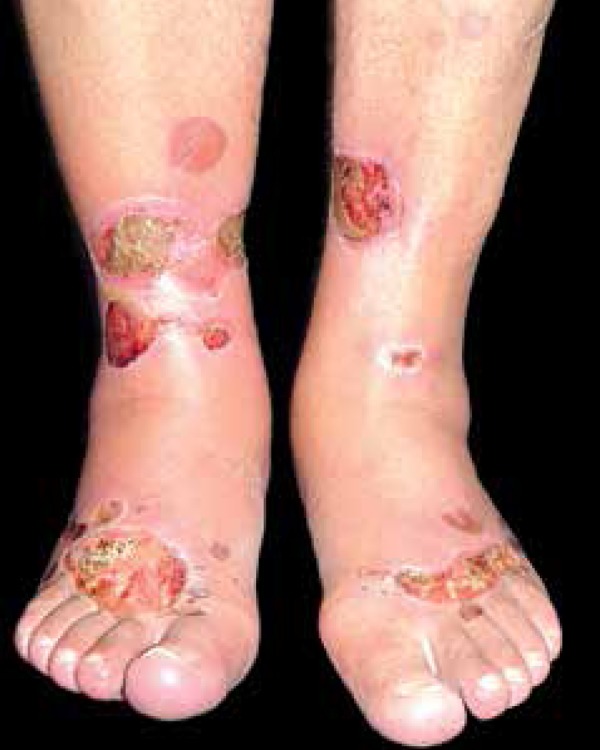
Classically, the so-called diabetic foot is a chronic ulcer that evolves after trauma or over a callus caused by changes in points with altered sensitivity due to diabetes neuropathy (6070%) (Figure 9). A much smaller proportion is linked to peripheral vascular ischemia (about 15%).
Figure 9.
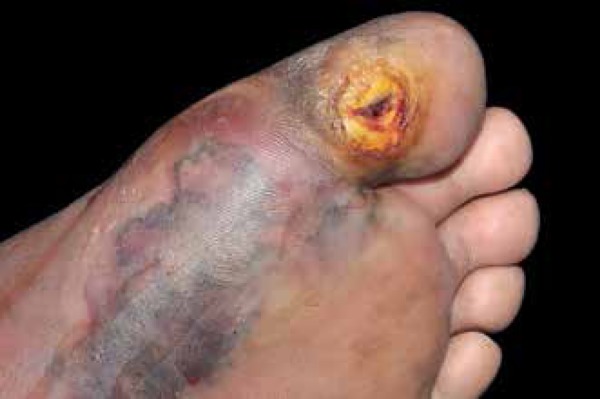
Diabetic foot may present a chronic ulcer on callus caused by changes in sensitivity associated with diabetic neuropathy and occasional ischemia
Photo: Department of Dermatology, Botucatu Medical School, UNESP
The causes can coexist and about 25% of diabetics may present foot ulcers during the development of the disease. 4,93
Ulcers are difficult to heal due to the underlying immunosuppression of the disease, hyperkeratotic borders and sometimes ischemia. In the US, the diabetic foot is responsible for 70% of lower limb amputations annually. 4,94
The treatment is performed according to the etiology. If the pulses are palpable, energetic therapeutic measures such as the debridement and dressing usually heal the wound in few weeks. On the other hand, no measures are effective in the presence of ischemia and surgical revascularization is crucial to the treatment in these cases. Secondary infections and osteomyelitis are factors that complicate the approach and systemic antibiotics should be evaluated in all cases.
There are specific guidelines to manage diabetic foot in a multidisciplinary approach that surpass the scope of this text. 95
MISCELLANEOUS
An ichthyosiform aspect may arise from changes of the skin in diabetes; it appear frequently in young subjects with insulindependent diabetes and appear associated with microangiopathy and duration of disease. Keratosis pilaris can also be observed and both appear to be associated with skin xerosis seen in these patients.
Rubeosis is a vascular erythema on the face and neck present in up to 60% of patients with diabetes, probably linked to the loss of the vasoconstrictor tone. It usually reflects poor glycemic control and is associated to peripheral neuropathy. In these patients, hyperglycemia can lead to a change in the microcirculation. It becomes clinically evident by facial venous dilatation. Rubeosis means microangiopathy, and it is prudent to assess patients for other microangiopathy such as retinopathy and nephropathy. Tight glucose control is the mainstay of treatment for this disease.
Yellow skin or carotenodermia is also related to inadequate glycemic control, which occurs either by carotenemia, as well as by increasing the glycosylation of collagen and dermal proteins.20 There is no treatment for this phenomenon.
Other conditions not necessarily related to the presence of diabetes are the eruptive xanthomas.94 These lesions are observed when there is a marked exacerbation of triglyceride levels (greater than 700mg/dL) caused by some triggering factor, among them one of the most common causes is the lack of DM control. The characteristic lesions may appear as papules in discrete or confluent domes, with waxy yellow centers and an erythematous base. Lesions may develop rapidly over the buttocks, elbows, and knees. They can be itchy and even painful. Eruptive xanthomas should be faced as a lifethreating disorder leading to acute pancreatitis that can be quickly resolved with proper correction of hypertriglyceridemia.83
Diabetes causes several changes in immunologic system, but especially the decrease in leukocyte chemotaxis and phagocytosis, in addition, impairment in vascular reaction leads to a significant deficiency of immune response that favors infections and delay their resolution.96
The most common fungal infections is candidiasis, especially vulvovaginal, balanopreputial, and angular stomatitis. These may be the first demonstration of the indirect presence of diabetes. Vulvovaginal candidiasis is almost universal among diabetic women in the long term and is a common cause of vulvar itching during periods of glycosuria. It comes with vulvar erythema and sometimes with white discharge. Treatment involves glycemic control in addition to topical or systemic treatment for specific fungal infection.81
Other common superficial mycoses in diabetics are extensive pityriasis versicolor and dermatophytoses (e.g. tinea corporis), which are associated to microangiopathy and poor glycemic control. Several opportunistic fungi infections are described in diabetics with poor glycemic control. A very serious condition, however rare, is mucormycosis, caused by Zygomycetes, from the order of Mucorales, which causes necrotic processes usually in the center of the face with a rapid progression, and with a high mortality rate. Early identification is essential for survival.97
Bacterial infections may be varied and severe as those caused by Staphylococcus or Pseudomonas. The infection may be mild or severe and may manifest as boils, abscesses or carbuncles. Recurrent erysipelas may also occur, as necrotizing/bullous erysipelas are common among diabetics. External otitis by Pseudomonas is also a serious condition in diabetics and may lead to mastoiditis, osteomyelitis of the temporal bone, damage to nerves and meninges, with a high mortality rate.77 Infections in diabetics have to be considered carefully and require hospitalization due to the sever compromise of immune response.
As autoimmune disorders are associated among themselves, diabetes (especially type 1 DM) can be associated to lupus erythematosus, alopecia areata and halo nevus.
VLTILIGO
Vitiligo is a chronic disease of autoimmune etiology that can manifest itself or, in most cases, associated with type 1 DM. It is characterized by an absence or dysfunction of melanocytes and appears as hypo/achromic spots surrounded by healthy skin whose size ranges from a few millimeters to large extensions, often located around holes, extensor regions, chest and abdomen.98
With an autosomal inheritance, it is estimated that vitiligo is manifested among 1% to 7% of all diabetic patients and only 0.2% to 1% of the general population.74 Although its pathogenesis is not entirely understood, it is suggested that its cause is polygenic, multifactorial, or a combination of autoimmune, genetic and neurohumoral factors due to the impairment of nerve cells release toxic substances harmful to melanocytes, leading to destruction of these cells while the pigment is forming.99 Environmental factors, such as infection or damage to the skin (Koebner phenomenon), may also contribute to the appearance of lesions.99
Moretti et al.95 found that the epidermis of vitiligo has a significant amount of cytokines in comparison with the healthy surrounding skin, suggesting that the production of these cytokines are involved in apoptosis of melanocytes process and depigmentation of the skin.96,99
Vitiligo can coexist with other disorders of autoimmune etiology, especially hormonal disorders (thyroiditis, adrenal insufficiency and hipoparatathyreoidism) as part of the polyglandular autoimmune syndrome whose clinical manifestations may appear in four different ways. The type 1 is the most common (1:20,000 individuals) and progresses with adrenal insufficiency, thyroiditis and type 1 DM, as well as atrophic gastritis, pernicious anemia, alopecia areata, celiac disease, myasthenia gravis and hypogonadism.97,98 So, when diagnosing vitiligo, physicians should be alert to the emergence of other autoimmune diseases, particularly type 1 DM.100-102
Although vitiligo is asymptomatic, the unpleasant discomfort and psychological stress can be considerable.11 Cosmetic treatment is an option to improve the quality of life.103 Skin camouflage and micropigmentation can be considered, as the treatment of vitiligo is unsatisfactory in general. Patients should be advised to avoid sun exposure and use broad spectrum sunscreens. In small and localized lesions topical corticosteroids are the first choice treatment, while for widespread vitiligo, treatment with narrowband ultraviolet light B is more effective. 81
PSORIASIS
Psoriasis is a chronic recurrent immunemediated inflammatory disease, with strong genetic component that affects 23% of the Caucasian population.104 It can occur at any age, although in most cases it develops before 40 years of age and is rare in children.105
Its emergence or worsening can often be triggered by emotional factors. Some studies have linked psoriasis with poorer quality of life, reduced life expectancy, bad employment and financial problems for the patient and family.106,107
The extent of skin involvement is variable, ranging from a few located plaques to widespread involvement. When the involvement is moderate to severe (>10% of their body surface area) it is often associated with psoriatic arthritis and metabolic syndrome, which is a set of risk factors for cardiovascular disease whose unifying factor is insulin resistance, conferring a pro-inflammatory and prothrombotic state.108-110
Several studies have evidenced the association of psoriasis with cardiovascular diseases and components of the metabolic syndrome (hypertension, obesity, dysglycemia or type 2 diabetes, dyslipidemia, fatty liver disease) and chronic kidney disease.107,110 Psoriasis patients often are overweight or obese in greater proportion.111-114 Furthermore, it was also observed higher mortality than the general population.115
Several theories were hypothesized to associate the concurrence of the components of metabolic syndrome, premature atherosclerosis and psoriasis. One of these suggests that common inflammatory pathways are involved in the pathophysiology of both, as the cytokine profile and the inflammatory cell infiltrate of T cells, macrophages and monocytes are observed in both conditions.114-116
The diagnosis of psoriasis is usually clinical, based on history and physical examination, but may be confirmed by the histopathological examination, which will reveal very characteristic aspects of the disease.107
Treatment of psoriasis depends on the clinical manifestations presented, varying from the simple application of topical medications in mild cases to more complex treatments for more severe cases. The response to treatment also varies greatly from one patient to another and the emotional component should not be overlooked. A healthy lifestyle, avoiding stress, will contribute to the improvement. Moderate sun exposure is of great help as keeping the skin well hydrated.
Although some drugs can negatively affect metabolic homeostasis by increasing cardiovascular risk, nonpharmacological interventions, such as nutrition education, smoking cessation and practice of physical activity associated with weight loss, can improve the response to treatments for psoriasis as well as reduce cardiovascular risk.
Even without complete remission of the disease, proper disease control promotes social rehabilitation of patients, improving the ability to work, and probably decreasing the risk of comorbidities.107
FINAL CONSIDERATIONS
Several cutaneous diseases are caused or may be influenced by systemic disorders and this knowledge is of major importance for the general practitioner.
DM is a highly prevalent systemic metabolic disease, whose cutaneous manifestations can help in the early diagnosis of the disease, thus reflecting glycemic control, systemic impairment or overall prognosis of the disease.
Adequate glycemic control and primary prevention of specific damage to internal organs should be promoted and reinforced by dermatologists, although many dermatological manifestations associated with DM are not necessarily related to glycemic levels nor definitely associated to the disease.
ACKNOWLEDGEMENTS
to the students Josiane Monção Andrella (Medical School of Botucatu - UNESP) and Michel Raineri Haddad (Medical School of São José do Rio Preto - FAMERP) for their help at various stages of the preparation of the manuscript.
Questions.
-
Diabetes mellitus is considered a modern epidemic. The disease affects about:
1.2% of adults
8.3% of adults
34.2% of adults
9.4% of adults
-
Acanthosis nigricans is not associated with:
certain malignancies such as gastric cancer
high doses of niacinamide
type A insulin resistance
nodular melanoma
-
Regarding bullosis diabeticorum, select the wrong answer:
diabetic blisters occurs in approximately 0.5% of diabetic patients
the preferred localization are the back and side of the hands and the arms
the diagnosis is made on clinical basis
the blisters are usually painless and nonpruritic
-
Diabetic dermopathy is (select the wrong answer):
the most common specific skin lesion in patients with diabetes
associated with cardiovascular disease
a pathognomonic sign for diabetes
typically located bilaterally in pretibial regions and distributed asymmetrically
-
The differential diagnosis of diabetic dermopathy includes (select the wrong answer):
Schamberg’s disease (progressive pigmented purpuric dermatitis)
stasis pigmented dermatitis, scarring lesions
factitious dermatitis
atopic dermatitis
-
Buschke´s scleredema (select the wrong answer):
is characterized by symmetrical and diffuse thickening with hardening of the skin
affects mainly the face, trunk, neck and upper limbs
is common in middle-aged men
is associated with diabetes mellitus in about 90% of cases.
-
Granuloma annulare is a benign and self-limited dermatitis that (select the wrong answer):
is characterized by papules which often assume an annular configuration
affects twice as many men than women
the generalized perforating form is characterized by umbilicated papules of about 4mm most commonly seen in children and young adults
disappeared lesions have about 40% chance to reappear
-
Necrobiosis lipoidica diabeticorum (select the wrong answer):
is not exclusive to diabetics because up to a third of cases occur in nondiabetic patients
approximately 35% of the lesions progress to ulceration
typical lesions start up in the pretibial areas
confirmed diagnosis of diabetes, abnormal plasma glucose or a family history of diabetes occur in 40% of patients
-
The diabetic foot is a severe complication that (select the wrong answer):
should be treated only by the dermatologist
is a chronic ulcer that evolves after trauma or over a callus caused by changes in points with altered sensitivity caused by diabetes neuropathy
In the US, is responsible for 70% of lower limb amputations annually
if the pulses are palpable, energetic therapeutic measures as the debridement and dressing usually heal the wound in a few weeks
-
Rubeosis is a vascular erythema on the face and neck that (select the wrong answer):
is observed in up to 60% of patients with diabetes
reflects poor glycemic control
becomes clinically evident by facial venous dilatation
the tight glucose control is not important for the treatment of the disease
-
The eruptive xanthomas (select the wrong answer):
are observed when there is a marked exacerbation of triglyceride levels (greater than 700mg/dL)
appear as papules in discrete or confluent domes, with waxy yellow centers and an erythematous base
the lesions may develop rapidly over the buttocks, elbows, and knees
do not resolve with proper correction of hypertriglyceridemia.
-
The most common fungal infections in diabetes mellitus (select the wrong answer):
is candidiasis, especially vulvovaginal, balanopreputial, and angular stomatitis
vulvovaginal candidiasis is almost universal among diabetic women in the long term and is a common cause of vulvar itching during periods of glycosuria
treatment does not need glycemic control, only topical or systemic treatment for Candida sp.
other common superficial mycoses in diabetics are extensive pityriasis versicolor and dermatophytoses
-
Which disease is not associated with diabetes mellitus?
lupus erythematosus
alopecia areata
acne conglobata
lichen planus
-
Vitiligo can coexist with other disorders of autoimmune etiology, especially:
thyroiditis
type 2 diabetes
hipoparatathyreoidism
alopecia areata
-
Patients with diabetes may have thickening and hardening of the skin (select the wrong answer):
of the dorsal region of the toes as well as the epidermis overlying the joints of the foot and toes
these changes are more common in type 1 patients
occur in up to 50% of the patients
the cause seems to be the glicosylation of proteins
-
The etiology of the granuloma annulare is unknown, but appears that is not involved with:
infections such as HIV
thyroid diseases
psoriasis
malignancy
-
Which body site is not compromised by the necrobiosis lipoidica diabeticorum?
foot
oral mucosa
abdomen
penis
-
Bacterial infections in diabetes may be varied and severe. Mark the wrong option:
are mainly caused by Staphylococcus or Pseudomonas
recurrent erysipelas may also occur, as necrotizing/bullous erysipelas are commoner among diabetics.
External otitis by Pseudomonas is a mild condition in diabetics
the bacterial infections in diabetics have to be considered carefully and require hospitalization due to the sever compromise of immune response
-
Which drug is not used in the treatment of the necrobiosis lipoidica diabeticorum?
intralesional injection and oral use of corticosteroids or topical threads under occlusion
clofazimine
tamoxifen
acetylsalicylic acid
-
Select the correct answer:
Diabetes mellitus is a high prevalent systemic metabolic disorder whose cutaneous manifestations can help in early diagnosis, as reflect either the glycemic control, organic compromise or overall disease prognosis.
Proper glycemic control and primary prevention of organspecific damage should be reinforced by dermatologists
many dermatologic manifestations during diabetes are not related to glycemic levels nor definitely associated to the disease
all the statements are correct
| Answer key | |||
|---|---|---|---|
| Palmar hyperhidrosis: clinical,
pathophysiological, diagnostic and therapeutic aspects.. 2016;91(6):716-25. | |||
| 1-C | 6-D | 11- B | 16- C |
| 2-B | 7-B | 12- A | 17- B |
| 3-D | 8-C | 13- B | 18- D |
| 4-D | 9-D | 14- C | 19- B |
| 5-A | 10- C | 15- C | 20- B |
|
Papers Information for all members: The EMC-D questionnaire is now available at the homepage of the Brazilian Annals of Dermatology: www.anaisdedermatologia.org.br. The deadline for completing the questionnaire is 30 days from the date of online publication. |
Footnotes
Conflict of interest: none
Financial support: none
Study conducted at the Departments of Dermatology and Clinical Medicine of the Faculdade de Medicina de Botucatu - Universidade Estadual Paulista “Júlio de Mesquita Filho” (UNESP) – Botucatu (SP), Brazil.
References
- 1.Idf.org IDF Diabetes Atlas. 6th ed. [2015 Jul 15]. Internet. Available from: http://www.idf.org/diabetesatlas/update2014.
- 2.Callen JP, Jorizzo JL, Bolognia JL, Piette WW, Zone JJ. Dermatological Signs of Internal Disease. 4th ed. Philadelphia: Saunders Elsevier; 2009. [Google Scholar]
- 3.Barbato MT, Criado PR, Silva AK, Averbeck E, Guerine MB, Sá NB. Association of acanthosis nigricans and skin tags with insulin resistance. An Bras Dermatol. 2012;87:97–104. doi: 10.1590/s0365-05962012000100012. [DOI] [PubMed] [Google Scholar]
- 4.Tamega Ade A, Aranha AM, Guiotoku MM, Miot LD, Miot HA. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25–31. doi: 10.1590/s0365-05962010000100003. [DOI] [PubMed] [Google Scholar]
- 5.Papa CM. Niacinamide and acanthosis nigricans. Arch Dermatol. 1984;120:1281–1281. [PubMed] [Google Scholar]
- 6.Romano G, Moretti G, Di Benedetto A, Giofrè C, Di Cesare E, Russo G, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101–106. doi: 10.1016/s0168-8227(97)00119-8. [DOI] [PubMed] [Google Scholar]
- 7.Kramer DW. Early or warning signs of impending gangrene in diabetes. Med J Rec. 1930;132:338–342. [Google Scholar]
- 8.Cantwell Jr AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42–44. [PubMed] [Google Scholar]
- 9.Mendes AL, Haddad Jr V. Caso para diagnostico. Bullosis Diabeticorum. An Bras Dermatol. 2007;82:94–96. [Google Scholar]
- 10.Basarab T, Munn SE, McGrath J, Russell Jones R. Bullosis diabeticorum. A case report and literature review. Clin Exp Dermatol. 1995;20:218–220. doi: 10.1111/j.1365-2230.1995.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 11.Paron NG, Lambert PW. Cutaneous manifestations of diabetes mellitus. Prim Care. 2000;27:371–383. doi: 10.1016/s0095-4543(05)70201-3. [DOI] [PubMed] [Google Scholar]
- 12.Rocca FF, Pereyra E. Phlyctenar lesions in the feet of diabetic patients. Diabetes. 1963;12:220–222. doi: 10.2337/diab.12.3.220. [DOI] [PubMed] [Google Scholar]
- 13.Allen GE, Hadden DR. Bullous lesions of the skin in diabetes (bullosis diabeticorum) Br J Dermatol. 1970;82:216–220. doi: 10.1111/j.1365-2133.1970.tb12427.x. [DOI] [PubMed] [Google Scholar]
- 14.Sehgal VN, Srivastava G, Aggarwal AK, Gupta M, Bhattacharya SN, Verma P. Noninsulindependent, type II diabetes mellitusrelated dermatoses: part II. Skinmed. 2011;9:302–308. [PubMed] [Google Scholar]
- 15.Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196–200. doi: 10.1046/j.1365-4362.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- 16.Kurwa A, Roberts P, Whitehead R. Concurrence of bullous and atrophic skin lesions in diabetes mellitus. Arch Dermatol. 1971;103:670–675. [PubMed] [Google Scholar]
- 17.Bernstein JE, Medenica M, Soltani K, Griem SF. Bullous eruption of diabetes mellitus. Arch Dermatol. 1979;115:324–325. [PubMed] [Google Scholar]
- 18.Parex I. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519–531. doi: 10.1016/s0190-9622(94)70058-3. [DOI] [PubMed] [Google Scholar]
- 19.Goodfield MJ, Millard LG, Harvey L, Jeffcoate WJ. Bullosis diabeticorum. J Am Acad Dermatol. 1986;15:1292–1294. doi: 10.1016/s0190-9622(86)80043-3. [DOI] [PubMed] [Google Scholar]
- 20.Shemer A, Bergman R, Linn S, Kantor Y, Friedman-Birnbaum R. Diabetic dermopathy and internal complications in diabetes mellitus. Int J Dermatol. 1998;37:113–115. doi: 10.1046/j.1365-4362.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Houck GM, Morgan MB. A reappraisal of the histologic findings of pigmented pretibial patches of diabetes mellitus. J Cutan Pathol. 2004;31:141–144. doi: 10.1111/j.0303-6987.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 22.Melin H. An atrophic circumscribed skin lesion in the lower extremities of diabetics. Acta Med Scand. 1964;176:1–75. [PubMed] [Google Scholar]
- 23.Binkley GW. Dermopathy in the diabetic syndrome. Arch Dermatol. 1965;92:625–634. [PubMed] [Google Scholar]
- 24.Mahajan S, Koranne RV, Sharma SK. Cutaneous manifestation of diabetes mellitus. Indian J Dermatol Venereol Leprol. 2003;69:105–108. [PubMed] [Google Scholar]
- 25.A Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, Sheikhi S, Eshrati B, Bastanhagh MH. Dermopathy and retinopathy in diabetes: is there an association? Dermatology. 2007;214:133–136. doi: 10.1159/000098572. [DOI] [PubMed] [Google Scholar]
- 26.Danowski TS, Sabeh G, Sarver ME, Shelkrot J, Fisher ER. Skin spots and diabetes mellitus. Am J Med Sci. 1966;251:570–575. doi: 10.1097/00000441-196605000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Lithner F. Cutaneous reactions of the extremities of diabetics to local thermal trauma. Acta Med Scand. 1975;198:319–325. doi: 10.1111/j.0954-6820.1975.tb19548.x. [DOI] [PubMed] [Google Scholar]
- 28.Wigington G, Ngo B, Rendell M. Skin blood flow in diabetic dermopathy. Arch Dermatol. 2004;140:1248–1250. doi: 10.1001/archderm.140.10.1248. [DOI] [PubMed] [Google Scholar]
- 29.Kiziltan ME, Benbir G, Akalin MA. Is diabetic dermopathy a sign for severe neuropathy in patients with diabetes mellitus? Nerve conduction studies and symptom analysis. Clin Neurophysiol. 2006;117:1862–1869. doi: 10.1016/j.clinph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Jelinek JE. Rifkin H, Porte D. Ellenberg and Rifkin's diabetes mellitus, theory and practice. New York: Elsevier; 1990. Skin disorders associated with diabetes mellitus; pp. 838–849. [Google Scholar]
- 31.Gouterman IH, Sibrack LA. Cutaneous manifestations of diabetes. Cutis. 1980;25:45–54. [PubMed] [Google Scholar]
- 32.Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults, part II: melanoma, seborrheic keratoses, acanthosis nigricans, melasma, diabetic dermopathy, tinea versicolor, and postinflammatory hyperpigmentation. Am Fam Physician. 2003;68:1963–1968. [PubMed] [Google Scholar]
- 33.Kroft EB, de Jong EM. Scleredema diabeticorum case series: successful treatment with UVA1. Arch Dermatol. 2008;144:947–948. doi: 10.1001/archderm.144.7.947. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Yamasaki Y, Watanabe T. Diabetic scleredema: a case report and biochemical analysis for glycosaminoglycan. J Dermatol. 1997;24:100–103. doi: 10.1111/j.1346-8138.1997.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 35.Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189–192. doi: 10.2337/diacare.6.2.189. [DOI] [PubMed] [Google Scholar]
- 36.Gervini RL, Lecompte SM, Pineda RC, Ruthner FG, Magnabosco EM, Silva LM. Escleredema de Buschke: relato de dois casos. An Bras Dermatol. 2002;77:465–472. [Google Scholar]
- 37.Emedicine.com PappasTaffer LK, Werth VP, Vinson RP, Libow LF, Elston DM. Scleredema. [2015 Nov 01]. Internet. Available from: www.emedicine.com/derm/topic385.htm.
- 38.Pitarch G, Torrijos A, Martínez-Aparicio A, Vilata JJ, Fortea JM. Escleredema de Buschke associado a diabetes mellitus. Estudio de cuatro casos. Actas Dermosifiliogr. 2005;96:46–49. doi: 10.1016/s0001-7310(05)73033-7. [DOI] [PubMed] [Google Scholar]
- 39.Sattar MA, Diab S, Sugathan TN, Sivanandasingham P, Fenech FF. Scleredema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465–468. doi: 10.1111/j.1464-5491.1988.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 40.Meguerditchian C, Jacquet P, Béliard S, Benderitter T, Valéro R, Carsuzza F, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481–484. doi: 10.1016/s1262-3636(07)70307-5. [DOI] [PubMed] [Google Scholar]
- 41.Carvalho JC, Costa TN, De Moura HH, Quintella LP, Carneiro S, Ramos-e-Silva M. Scleredema Adultorum of Buschke. Skinmed. 2014;12:337–340. [PubMed] [Google Scholar]
- 42.Barde C, Masouyé I, Saurat JH, Le Gal FA. Scleroedema adultorum Buschke in a diabetic subject: intravenous immunoglobulin therapy. Ann Dermatol Venereol. 2009;136:360–363. doi: 10.1016/j.annder.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 43.Mokta J, Sharma B, Patial RK, Prasher BS, Prasher N. Systemic manifestations in scleredema of Buschke. J Assoc Physicians India. 2001;49:1033–1034. [PubMed] [Google Scholar]
- 44.Oyama N, Togashi A, Kaneko F, Yamamoto T. Two cases of scleredema with pituitaryadrenocortical neoplasms: an underrecognized skin complication. J Dermatol. 2012;39:193–195. doi: 10.1111/j.1346-8138.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- 45.Parmar RC, Bavdekar SB, Bansal S, Doraiswamy A, Khambadkone S. Scleredema adultorum. J Postgrad Med. 2000;46:91–93. [PubMed] [Google Scholar]
- 46.Mattos e Dinato SM, Costa GL, Dinato MC, Sementilli A, Romiti N. Escleredema de Buschke associado ao diabetes melito tipo 2: relato de caso e revisão da literatura. Arq Bras Endocrinol Metab. 2010;54:852–855. doi: 10.1590/s0004-27302010000900012. [DOI] [PubMed] [Google Scholar]
- 47.Kövary PM, Vakilzadeh F, Macher E, Zaun H, Merk H, Goerz G. Monoclonal gammopathy in scleredema: observations in three cases. Arch Dermatol. 1981;117:536–539. doi: 10.1001/archderm.1981.01650090018016. [DOI] [PubMed] [Google Scholar]
- 48.Angeli-Besson C, Koeppel MC, Jacquet P, Andrac L, Sayag J. Electronbeam therapy in scleredema adultorum with associated monoclonal hypergammaglobulinaemia. Br J Dermatol. 1994;130:394–397. doi: 10.1111/j.1365-2133.1994.tb02940.x. [DOI] [PubMed] [Google Scholar]
- 49.Turchin I, Adams SP, Enta T. Answer to dermacase. Can Fam Physician. 2003;1093;49:1089–1089. [PMC free article] [PubMed] [Google Scholar]
- 50.Ray V, Boisseau-Garsaud AM, Ray P, Pont F, Lin L, Hélénon R, et al. Obesity persistent scleredema: study of 49 cases. Ann Dermatol Venereol. 2002;129:281–285. [PubMed] [Google Scholar]
- 51.Graff R. Discussion of scleredema adultorum. Arch Derm. 1968;98:319–320. doi: 10.1001/archderm.98.3.319. [DOI] [PubMed] [Google Scholar]
- 52.Ulmer A, Schaumburg-Lever G, Bauer J, Kötter I, Fierlbeck G. Scleredema adultorum Buschke. Case report and review of the literature. Hautarzt. 1998;49:48–54. doi: 10.1007/s001050050700. [DOI] [PubMed] [Google Scholar]
- 53.Cron RQ, Swetter SM. SM. Scleredema revisited. Clin Pediatr (Phila) 1994;33:606–610. doi: 10.1177/000992289403301006. [DOI] [PubMed] [Google Scholar]
- 54.Martín C, Requena L, Manrique K, Manzarbeitia FD, Rovira A. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273–560273. doi: 10.1155/2011/560273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horger M, Fierlbeck G, Kuemmerle-Deschner J, Tzaribachev N, Wehrmann M, Claussen CD, et al. MRI findings in deep and generalized morphea (localized scleroderma) AJR Am J Roentgenol. 2008;190:32–39. doi: 10.2214/AJR.07.2163. [DOI] [PubMed] [Google Scholar]
- 56.Cole GW, Handler SJ, Burnett K. The ultrasonic evaluation of skin thickness in scleredema. J Clin Ultrasound. 1981;9:501–503. doi: 10.1002/jcu.1870090907. [DOI] [PubMed] [Google Scholar]
- 57.Kurihara Y, Kokuba H, Furue M. Case of diabetic scleredema: diagnostic value of magnetic resonance imaging. J Dermatol. 2011;38:693–696. doi: 10.1111/j.1346-8138.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 58.Conde Fernandes I, Sanches M, Velho G, Lobo I, Alves R, Selores M. Scleromyxedema vs scleredema: a diagnostic challenge. Eur J Dermatol. 2011;21:822–823. doi: 10.1684/ejd.2011.1493. [DOI] [PubMed] [Google Scholar]
- 59.Gruson LM, Franks Jr A. Scleredema and diabetic sclerodactyly. Dermatol Online J. 2005;11:3–3. [PubMed] [Google Scholar]
- 60.Thumpimukvatana N, Wongpraparut C, Lim HW. Scleredema diabeticorum successfully treated with ultra¬violet A1 phototherapy. J Dermatol. 2010;37:1036–1039. doi: 10.1111/j.1346-8138.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 61.Alsaeedi SH, Lee P. Treatment of scleredema diabeticorum with tamoxifen. J Rheumatol. 2010;37:2636–2637. doi: 10.3899/jrheum.100561. [DOI] [PubMed] [Google Scholar]
- 62.Dahl MV. Granuloma annulare. In: Freedberg IM, Eisen AZ, Wolff K, Austin KF, Goldsmith LA, Katz SL, editors. Fitzpatrick's Dermatology in General Medicine. 5th ed. New York: McGrawHill; 1999. pp. 1152–1157. [Google Scholar]
- 63.Dabski K, Winkelmann RK. Generalized granuloma annulare. Clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39–47. doi: 10.1016/s0190-9622(89)70005-0. [DOI] [PubMed] [Google Scholar]
- 64.Cohen PR. Granuloma annulare associated with malignancy. South Med J. 1997;90:1056–1059. doi: 10.1097/00007611-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 65.Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364–368. doi: 10.1159/000246297. [DOI] [PubMed] [Google Scholar]
- 66.Erkek E, Karaduman A, Bükülmez G, Sentürk N, Ozkaya O. An unusual form of generalized granuloma annulare in a patient with insulindependent diabetes mellitus. Acta Derm Venereol. 2001;81:48–50. doi: 10.1080/00015550121061. [DOI] [PubMed] [Google Scholar]
- 67.Buendía-Eisman A, Ruiz-Villaverde R, Blasco-Melguizo J, Serrano-Ortega S. Generalized annular granuloma: response to isotretinoin. Int J Dermatol. 2003;42:321–322. doi: 10.1046/j.1365-4362.2003.01799_4.x. [DOI] [PubMed] [Google Scholar]
- 68.Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729–1734. [PubMed] [Google Scholar]
- 69.Sahin MT, Türel-Ermertcan A, Oztürkcan S, Türkdogan P. Generalized granuloma annulare in a patient with type II diabetes mellitus: successful treatment with isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:111–114. doi: 10.1111/j.1468-3083.2006.01337.x. [DOI] [PubMed] [Google Scholar]
- 70.Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689–703. [PubMed] [Google Scholar]
- 71.Lowitt MH, Dover JS. Necrobiosis lipoidica. J Am Acad Dermatol. 1991;25:735–748. doi: 10.1016/s0190-9622(08)80961-9. [DOI] [PubMed] [Google Scholar]
- 72.Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum: a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272–281. doi: 10.1001/archderm.93.3.272. [DOI] [PubMed] [Google Scholar]
- 73.Yosipovitch G, Hodak E, Vardi P, Shraga I, Karp M, Sprecher E. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506–509. doi: 10.2337/diacare.21.4.506. [DOI] [PubMed] [Google Scholar]
- 74.Garza-Elizondo MA, Diaz-Jouanen E, Franco-Casique JJ, Alarcón-Segovia D. Joint contractures and sclerodermalike skin changes in the hands of insulindependent juvenile diabetes. J Rheumatol. 1983;10:797–800. [PubMed] [Google Scholar]
- 75.Jelinek JE. The skin in diabetes. Diabet Med. 1993;10:201–213. doi: 10.1111/j.1464-5491.1993.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 76.Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med. 2008;75:772, 774, 776-7. doi: 10.3949/ccjm.75.11.772. [DOI] [PubMed] [Google Scholar]
- 77.Sibbald RG, Landolt SJ, Toth D. Skin and diabetes. Endocrinol Metab Clin North Am. 1996 Jun;25(2):463–472. doi: 10.1016/s0889-8529(05)70334-0. [DOI] [PubMed] [Google Scholar]
- 78.Petzelbauer P, Wolff K, Tappeiner G. Necrobiosis lipoidica: treatment with systemic corticoids. Br J Dermatol. 1992;126:542–545. doi: 10.1111/j.1365-2133.1992.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 79.Oumeish YO. Skin disorders in patients with diabetes. Clin Dermatol. 2008;26:235–242. doi: 10.1016/j.clindermatol.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 80.Dahl MV, Ullman S, Fisher I. Direct immunofluorescence of granuloma annulare and necrobiosis lipoidica. Clin Res. 1976;24:95a–95a. [Google Scholar]
- 81.Quimby SR, Muller SA, Schroeter AL. The cutaneous immunopathology of necrobiosis lipoidica diabeticorum. Arch Dermatol. 1988;124:1364–1371. [PubMed] [Google Scholar]
- 82.Ullman S, Dahl MV. Necrobiosis lipoidica. An immunofluorescence study. Arch Dermatol. 1977;113:1671–1673. [PubMed] [Google Scholar]
- 83.Beattie PE, Dawe RS, Ibbotson SH, Ferguson J. UVA1 phototherapy for treatment of necrobiosis lipoidica. Clin Exp Dermatol. 2006;31:235–238. doi: 10.1111/j.1365-2230.2005.02059.x. [DOI] [PubMed] [Google Scholar]
- 84.Nieboer C, Kalsbeek GL. Direct immunofluorescence studies in granuloma annulare, necrobiosis lipoidica and granulomatosis disciformis Miescher. Dermatologica. 1979;158:427–432. doi: 10.1159/000250793. [DOI] [PubMed] [Google Scholar]
- 85.Dwyer CM, Dick D. Ulceration in necrobiosis lipoidica: a case report and study. Clin Exp Dermatol. 1993;18:366–369. doi: 10.1111/j.1365-2230.1993.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 86.Bauer M, Levan NE. Diabetic dermangiopathy. A spectrum including pretibial pigmented patches and necrobiosis lipoidica diabeticorum. Br J Dermatol. 1970;83:528–535. doi: 10.1111/j.1365-2133.1970.tb15736.x. [DOI] [PubMed] [Google Scholar]
- 87.Ahmed I, Goldstein B. Diabetes mellitus. Clin Dermatol. 2006;24:237–246. doi: 10.1016/j.clindermatol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Ferringer T, Miller 3rd F. Cutaneous manifestations of diabetes mellitus. Dermatol Clin. 2002;20:483–492. doi: 10.1016/s0733-8635(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 89.Cohen O, Yaniv R, Karasik A, Trau H. Necrobiosis lipoidica and diabetic control revisited. Med Hypotheses. 1996;46:348–350. doi: 10.1016/s0306-9877(96)90184-x. [DOI] [PubMed] [Google Scholar]
- 90.Tokura Y, Mizushima Y, Hata M, Takigawa M. Necrobiosis lipoidica of the glans penis. J Am Acad Dermatol. 2003;49:921–924. doi: 10.1016/s0190-9622(03)00443-2. [DOI] [PubMed] [Google Scholar]
- 91.Nguyen K, Washenik K, Shupack J. Necrobiosis lipoidica diabeticorum treated with chloroquine. J Am Acad Dermatol. 2002;46:S34–S36. doi: 10.1067/mjd.2002.104969. [DOI] [PubMed] [Google Scholar]
- 92.Alexis AF, Strober BE. Offlabel dermatologic uses of antiTNFa therapies. J Cutan Med Surg. 2005;9:296–302. doi: 10.1007/s10227-005-0110-7. [DOI] [PubMed] [Google Scholar]
- 93.Tabor CA, Parlette EC. Cutaneous manifestations of diabetes. Signs of poor glicemy control or newonset disease. Postgrad Med. 2006;119:38–44. doi: 10.1080/00325481.2006.11446049. [DOI] [PubMed] [Google Scholar]
- 94.Ngo BT, Hayes KD, DiMiao DJ, Srinivasan SK, Huerter CJ, Rendell MS. Cutaneous manifestations of diabetic microangiopathy. Am J Clin Dermatol. 2005;6:225–237. doi: 10.2165/00128071-200506040-00003. [DOI] [PubMed] [Google Scholar]
- 95.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, et al. American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision) J Foot Ankle Surg. 2006;45:S1–66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 96.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 97.Arnáiz-García ME, Alonso-Peña D, González-Vela Mdel C, García-Palomo JD, Sanz-Giménez-Rico JR, Arnáiz-García AM. Cutaneous mucormycosis: report of five cases and review of the literature. J Plast Reconstr Aesthet Surg. 2009;62:e434–e441. doi: 10.1016/j.bjps.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 98.Passeron T, Ortonne JP. Physiopathology and genetics of vitiligo. J Autoimmun. 2005;25:63–68. doi: 10.1016/j.jaut.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesion. Pigment Cell Res. 2002;15:87–92. doi: 10.1034/j.1600-0749.2002.1o049.x. [DOI] [PubMed] [Google Scholar]
- 100.Olasode A. Why vitiligo in diabetes. Egypt Dermatol Online J. 2005;1:8–8. [Google Scholar]
- 101.Huang CL, Nordlund JJ, Boissy R. Vitiligo: a manifestation of apoptosis? Am J Clin Dermatol. 2002;3:301–308. doi: 10.2165/00128071-200203050-00001. [DOI] [PubMed] [Google Scholar]
- 102.Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: Immunogenetics and longterm followup. J Clin Endocrinol Metab. 2003;88:2983–2992. doi: 10.1210/jc.2002-021845. [DOI] [PubMed] [Google Scholar]
- 103.Forschner T, Buchholtz S, Stockfleth E. Current state of vitiligo therapyevidencebased analysis of the literature. J Dtsch Dermatol Ges. 2007;5:467–475. doi: 10.1111/j.1610-0387.2007.06280.x. [DOI] [PubMed] [Google Scholar]
- 104.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012 Dec;44(12):1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gisondi P, Galvan A, Idolazzi L, Girolomoni G. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne) 2015 Jan;21(1):1. doi: 10.3389/fmed.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gisondi P, Girolomoni G. Impact of TNF? antagonists on the quality of life in selected skin diseases. G Ital Dermatol Venereol. 2013;148:243–248. [PubMed] [Google Scholar]
- 107.Sociedade Brasileira de Dermatologia. Consenso Brasileiro de Psoríase 2012 . Guias de Avaliação e Tratamento. 2. Rio de Janeiro: Sociedade Brasileira de Dermatologia; 2012. [Google Scholar]
- 108.Grundy SM, Brewer Jr HB, Cleeman JI, Smith Jr SC, Lenfant C, American Heart AssociationNational Heart.Lung.and Blood Institute Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 109.Gisondi P, Girolomoni G. Cardiometabolic comorbidities and the approach to patients with psoriasis. Actas Actas Dermosifiliogr. 2009;100:14–21. doi: 10.1016/s0001-7310(09)73373-3. [DOI] [PubMed] [Google Scholar]
- 110.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–328. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 111.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and metaanalysis of observational studies. Nutr Diabetes. 2012 Dec;3(2):e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: nurses' health study II. Arch Intern Med. 2007;167:1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 113.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a populationbased study. Arch Dermatol. 2007;143:1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 114.Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and comorbid conditions. J Invest Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 115.Boehncke WH, Boehncke S, Tobin AM, Kirby B. The 'psoriatic march': a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20:303–307. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 116.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]


