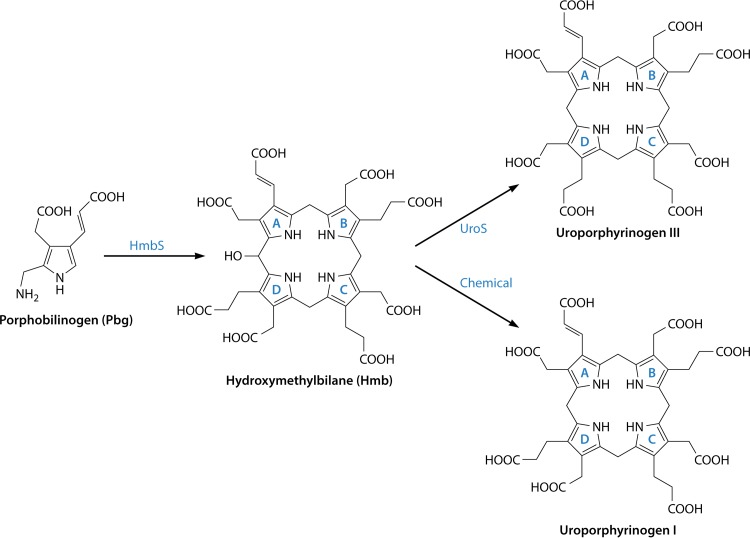
FIG 7.
Transformation of porphobilinogen into uroporphyrinogen III. Four molecules of porphobilinogen are deaminated and polymerized in an ordered sequential fashion (rings A to D) into a linear tetrapyrrole called hydroxymethylbilane by the action of hydroxymethylbilane synthase. The unstable bilane is acted upon by the enzyme uroporphyrinogen synthase, which inverts ring D and cyclizes the macrocycle to give the type III isomer of uroporphyrinogen. In the absence of the enzyme, hydroxymethylbilane spontaneously cyclizes to give the type I isomer.