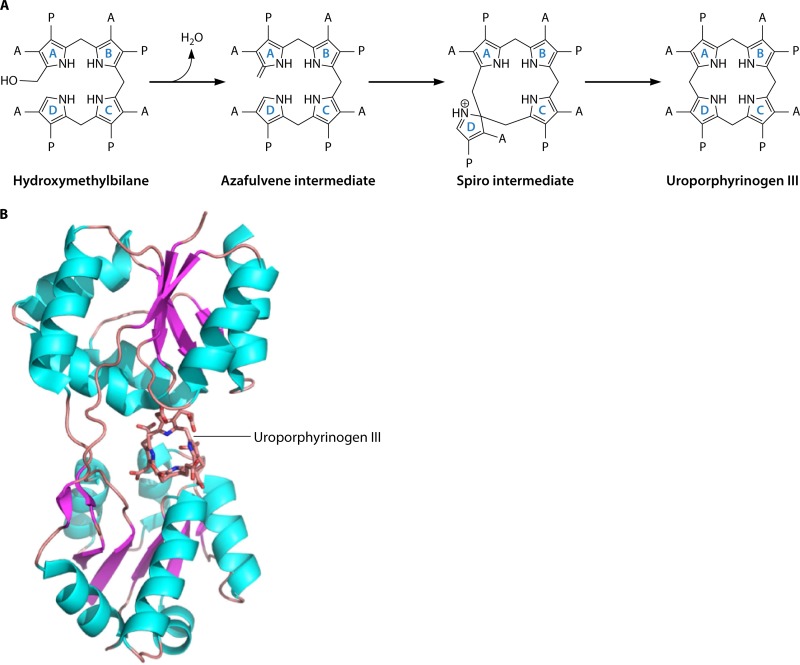
FIG 9.
Mechanism and structure of uroporphyrinogen III synthase (UroS). (A) Mechanism of action of UroS. The transformation of hydroxymethylbilane into uroporphyrinogen III is thought to proceed via a spiro intermediate, which is itself formed from an azafulvene intermediate generated from the loss of the hydroxyl group. Rearrangement of the spiro intermediate is then able to produce the type III isomer of uroporphyrinogen. The acetate and propionate side chains are designated A and P, respectively. (B) Structure of UroS. The structure of uroporphyrinogen III synthase from T. thermophilus, together with its product uroporphyrinogen III, is shown in cartoon format. The 252 amino acid residues of the enzyme are folded into two α/β-domains with a large active site formed at their juncture. There are a few highly conserved amino acid residues found in the active site, suggesting that the binding of the substrate in the correct orientation promotes the chemistry outlined in panel A.