Figure 5. 3E10EN mediates autocatalytic, tumor-targeted delivery of nanoparticles.
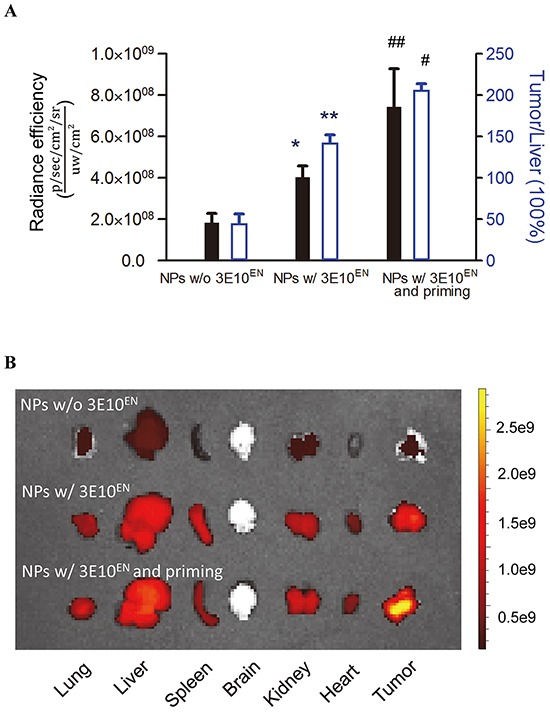
IR780-loaded nanoparticles with or without 3E10EN conjugation were administered intravenously to 4T1 tumor-bearing mice. Twenty-four hours later, tumors were excised and imaged using an IVIS imaging system. Naked NPs were observed to localize into a range of tissues. By contrast, 3E10EN-conjugated NPs showed a pattern of preferential uptake into tumors and a 2.3 fold increase in tumor localization compared to naked NPs. In addition, priming treatments with 3E10EN/DOX-NPs significantly enhanced tumor delivery of the nanoparticles. The average amount of nanoparticles in tumors from mice that received priming treatments was 1.8 times greater than the amount in tumors from mice without priming. With priming, the accumulation of nanoparticles in tumors was 4.1 times higher than that in the liver, compared to 0.5 times for mice that received treatment with naked NPs. Quantitative analysis of the accumulation of indicated nanoparticles in tumors (n=4) is shown in A. * and # represent statistical analyses between the NPs w/ 3E10EN group and the NPs w/o 3E10EN group, and between the NPs w/ 3E10EN and priming group and the NPs w/ 3E10EN group, respectively. * and #: P < 0.05, ** and ##: P < 0.01. Representative IVIS images of the bio-distribution of nanoparticles are shown in B.
