Abstract
Long-term in vivo imaging in adult zebrafish (i.e., 1–24 h) has been limited by the fact that regimens for long-term anesthesia in embryos and larvae are ineffective in adults. Here, we examined the potential for dynamic administration of benzocaine to enable long-term anesthesia in adult zebrafish. We developed a computer-controlled perfusion system comprised of programmable peristaltic pumps that enabled automatic exchange between anesthetic and system water. Continuous administration of benzocaine in adult zebrafish resulted in a mean time to respiratory arrest of 5.0 h and 8-h survival of 14.3%. We measured characteristic sedation and recovery times in response to benzocaine, and used them to devise an intermittent dosing regimen consisting of 14.5 min of benzocaine followed by 5.5 min of system water. Intermittent benzocaine administration in adult zebrafish resulted in a mean time to respiratory arrest of 7.6 h and 8-h survival of 71.4%. Finally, we performed a single 24-h trial and found that intermittent dosing maintained anesthesia in an adult zebrafish over the entire 24-h period. In summary, our studies demonstrate the potential for dynamic administration of benzocaine to enable prolonged anesthesia in adult zebrafish, expanding the potential for imaging in adult physiologies that unfold over 1–24 h.
Keywords: : anesthesia, imaging, fin, regeneration, long-term, MS-222, tricaine
Introduction
Zebrafish represent a powerful model for biomedical research due in large part to their optical properties and amenability to in vivo imaging. Over the last several decades, a growing number of studies have demonstrated the value in using juvenile and adult zebrafish to examine physiologies beyond early developmental periods. Such physiologies include processes associated with postembryonic development (e.g., aspects of skeletal development, adult pigment pattern formation, appearance of median and pelvic fins1), disease (e.g., cancer2), and regeneration (including bone,3 heart,4 and central nervous system5). As the use of juvenile and adult zebrafish increases, there is a need to adapt or refine methods that have been optimized for early embryonic development, which may not be suited for later ontogenetic windows. One such need is the necessity for reliable regimens for prolonged anesthesia in adult zebrafish6–10 to facilitate long-term in vivo imaging.
The most commonly used anesthetic in zebrafish is tricaine methanesulfonate (MS-222).9 MS-222 is a known inhibitor of sodium channels, and its efficacy in sedating or anesthetizing zebrafish is attributable to multiple mechanisms including blocking sodium action potentials in muscle (preventing muscle contractions), depressing central nervous system activity, and/or inhibiting nerve conduction.6,11,12 In zebrafish embryos (e.g., during the first 72 h of development), MS-222 is effective in immobilizing animals for 24–48 h or more.13,14 However, in adults, MS-222 forces the zebrafish into respiratory arrest in ∼20 min or less.6 Efforts to extend this anesthetic duration in adult zebrafish by administering different MS-222 concentrations have been largely ineffective as doses below ∼0.01% do not result in sufficient sedation or immobility, despite retaining the potential to induce respiratory arrest.6 The lack of reliable methods for long-term anesthesia in adult zebrafish has impeded insights into biological processes within physiologies whose dynamics unfold in the span of 1–24 h.
Anesthetic durations in adult zebrafish may be extended through two strategies. The first is the use of alternative anesthetic compounds.6–10 Huang et al. developed an anesthetic cocktail of MS-222 and isoflurane that extended the anesthetic period in adult animals from ∼3 to 12 min with MS-222 to ∼40–50 min.6 As an alternative to MS-222 cocktails, structural isomers of MS-222 might also extend anesthetic duration in adult animals.15–17 In this context, benzocaine is a structural analog of MS-222 with similar pharmacodynamic characteristics in other aquatic species, yet with less tendency to acidify the water.17,18 To date, the efficacy of benzocaine as a long-term anesthetic agent in adult zebrafish has yet to be explored.7 A second strategy to prolong anesthetic periods in adult zebrafish is to modulate anesthetic stage by dynamically altering anesthetic concentration. Given the rapid induction and moderate recovery times of benzocaine in other aquatic species,15,17 we postulated it may be ideal for use in a dynamic administration system that intermittently administered anesthetic and fresh water to prolong anesthetic periods.
In this study, our goals were twofold: (1) develop a dynamic perfusion system for imaging in adult zebrafish, and (2) examine the potential for continuous and intermittent administration of benzocaine to maintain long-term anesthesia in adult zebrafish.
Materials and Methods
Animal care
All studies were performed on an approved protocol in accordance with the University of Washington Institutional Animal Care and Use Committee (IACUC). Zebrafish were housed at a temperature of 27°C–29°C on a 14:10 h light:dark photoperiod. Studies were conducted in mixed sex adult (∼30–40 mm standard length) wild-type zebrafish (Aquatic Research Organisms, Hampton, NH) and transgenic fluorescent reporter strains [Tg(Sp7:EGFP)b1212 and Tg(7 × TCF-Xla.Siam:GFP)ia4]19,20 that enabled assessment of image quality during anesthetic procedures. All fish were housed in plastic tanks on a commercial recirculating aquaculture system. System water quality was as follows: pH: 7.5–7.8; Un-ionized Ammonia: <0.02 mg/L; Nitrite: <0.1 mg/L; Nitrate: <20 mg/L; Conductivity: 500–1000 μS; Total Hardness: 100–350 mg/L; Alkalinity: 30–150 mg/L. Fish were fed a commercial diet (Zeigler, Gardners, PA) twice per day to satiation. Health status of the colony was monitored by evaluation of monthly population, morbidity, and mortality rates and subsequent histopathological evaluation.
Anesthetic preparation
MS-222 (E10521; Sigma-Aldrich, St. Louis, MO) was dissolved in deionized water at a concentration of 4 mg/mL, adjusted with sodium hydroxide,9 and this stock solution was mixed with system water to create a 0.02% (200 mg/L) solution with a pH of 7–8. For preparation of benzocaine (E1501; Sigma-Aldrich), anesthetic was dissolved in 70% ethanol at a concentration of 100 mg/mL, and this stock solution was mixed with system water to create a 0.0035% (35 mg/L) solution with a pH of ∼7.6. This concentration of benzocaine was selected and employed as a standard dose for all our studies as it was the lowest dose that reliably provided maintenance of stage 3 anesthesia after initial induction with buffered MS-222.
Anesthetic system
For construction of the imaging chamber, a three-dimensional (3D) model was designed using Pro/ENGINEER (PTC, Needham, MA) and constructed in PLA (MakerBot Industries, New York City, NY) plastic using a MakerBot Replicator® 2 3D printer (MakerBot Industries). FCStd files compatible with the open-source 3D CAD modeling program FreeCAD (www.freecadweb.org) are included as supplementary data; (Supplementary Data are available online at www.liebertpub.com/zeb). The imaging chamber consisted of a 110 × 40 × 16 mm rectangular dish with a depression in the bottom for insertion of a glass slide to mount the fish. Two flow inlets and a single flow outlet were created using 1/16″ tubing connector bulkheads (EW-40622-49; Cole-Parmer, Vernon Hills, IL) and fixed to the chamber using pharmaceutical cyanoacrylate (Vetbond™; 3M, St. Paul, MN). Vinyl tubing (EW-40622-23; Cole-Parmer) was used to connect each of the two inlets and the outlet to low flow peristaltic pumps (YO-73160; Cole-Parmer). Total length (∼6 ft) and inner diameter (1/16′′) of the tubing were minimized to reduce the volume of fluid between the heated reservoirs and the chamber. The two inflow pumps were powered by a computer programmable powerstrip (PowerUSB-Basic, Plano, TX). The first pump was fed by a large reservoir containing 0.0035% benzocaine; the second pump was fed by a reservoir containing system water. Both reservoirs were heated to 29°C with the use of submersible heaters (AquaTop, Brea, CA). The outflow pump fed water into a waste beaker. The flow rate was held at 3.9 mL/min for each inlet and ≥3.9 mL/min for the outlet.
Anesthetic trials
For each anesthetic trial, a single fish was initially anesthetized in buffered MS-222, positioned on its side on a glass slide, and the tail fin was adhered to the glass slide using pharmaceutical grade cyanoacrylate (Vetbond™; 3M). The latter step facilitated imaging and may be omitted (or adapted for imaging in other anatomical sites). Due to the greater induction time associated with using a 0.0035% benzocaine solution, we used a buffered 0.02% solution of MS-222 for the initial induction to ensure rapid anesthetic depth to stage 3 to induce the immobility and provide analgesia required to adhere the tail fin to the glass slide. Once the tissue adhesive dried, the slide (with fish on top) was placed in the chamber, and the chamber was filled with benzocaine solution up to the inflow/outflow line. For evaluation of anesthetic stage, we used criteria adapted from Laycock21 and Noga22 and described in Table 1. Reflex response was assessed by acoustic startle.23 In cases where attributes from two stages were exhibited, we assessed the stage as the mean of the two stages in question. Personnel were present to observe fish at all times. Respiratory rate (RR) was measured every 15–20 min by visual observation of opercular movements. Previous studies have demonstrated that respiratory arrest quickly leads to death,6,24 thus survival time was assessed as the time in which a respiratory rate of zero was first observed. The pH and alkalinity in the reservoirs was measured using pH meters and alkalinity test kits (LM4482 and LM4491; Pentair Aquatic Eco-Systems, Inc., Apopka, FL). Measurement of these parameters within the chamber was performed using water quality test strips (H27448 and H27456; Pentair Aquatic Eco-Systems, Inc.).
Table 1.
Description of Anesthetic Stages
| Anesthetic stage | Description |
|---|---|
| Stage 1 | beginning of anesthesia, initial hyperactivity followed by decreased movement and respiration rate, strong reflex response following audible startle |
| Stage 2 | continued reduction in movement, loss of consciousness, equilibrium, and muscle tone, further decrease in voluntary respiration rate, reduced reflex response following audible startle |
| Stage 3 | immobility, loss of reflex response following audible startle, further reduction in voluntary respiration to respiratory arrest |
| Stage 4 | respiratory arrest to death |
Description of criteria used to evaluate anesthetic stage.
Statistical analysis
Kaplan–Meier survival curves were constructed in Prism (Graphpad Software, La Jolla, CA). The exponential Greenwood formula was used to calculate confidence intervals. Cox proportional hazards regression analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria). Comparisons between two groups were performed using a t-test assuming equal variances and a two-tailed distribution, with paired t-tests performed when appropriate. p < 0.05 was considered statistically significant. Unless noted, all data are presented as mean ± SE.
Results
A computer-controlled perfusion system enables dynamic modulation of anesthetic plane
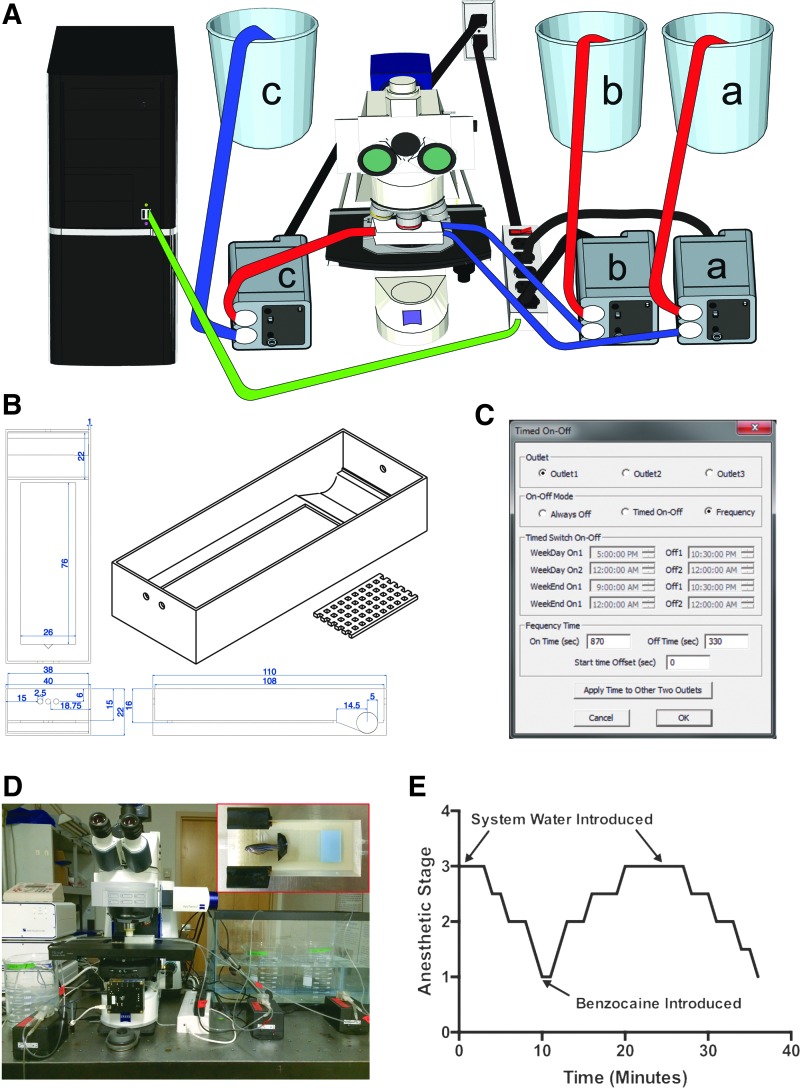
We constructed a programmable perfusion system consisting of computer-controlled peristaltic pumps that intermittently delivered heated benzocaine solution or system water to a 3D printed imaging chamber (Fig. 1A–D). Within the chamber, a rounded depression with a grid placed over it facilitated the movement of water past the gills of a fish placed in a lateral recumbent position, enabling imaging on the lateral surface. Dosing regimens ranging from continuous perfusion to intermittent administration of different periods could be programmed and administered automatically over long durations. In addition, the user could superimpose additional changes between anesthetic and system water by selecting the appropriate pump from the computer interface. We anesthetized adult zebrafish to stage 3 and intermittently administered system water and 0.0035% benzocaine (Fig. 1E). Following administration of system water, the animals exhibited brief recovery to stage 1, descent into stage 3 after administration of benzocaine, and rerecovery following reperfusion with system water.
FIG. 1.
Depiction of the anesthesia system and intermittent dosing regimen. (A) Schematic of all system components with labels; a: anesthetic water supply and pump; b: system water supply and pump; c: waste water and pump. Red tubes indicate flow toward pump and blue tubes indicate flow away from pump. Green cable is a USB cord controlling timed operation of power strip via a computer. Black cables are standard power cords. (B) CAD drawings of imaging chamber. Dimensions are in millimeters. (C) Screen shot of programmable power strip interface (settings shown for Outlet 1). (D) Photograph of the system with all the same components as in (A). Inset: image of the three-dimensional printed anesthetic chamber with fish mounted to a glass slide. (E) Representative example of intermittent dosing trial from a single fish to determine time to stage 3 sedation and subsequent recovery.
Continuous administration of benzocaine prolongs time to respiratory arrest relative to established anesthetic regimens in adult zebrafish
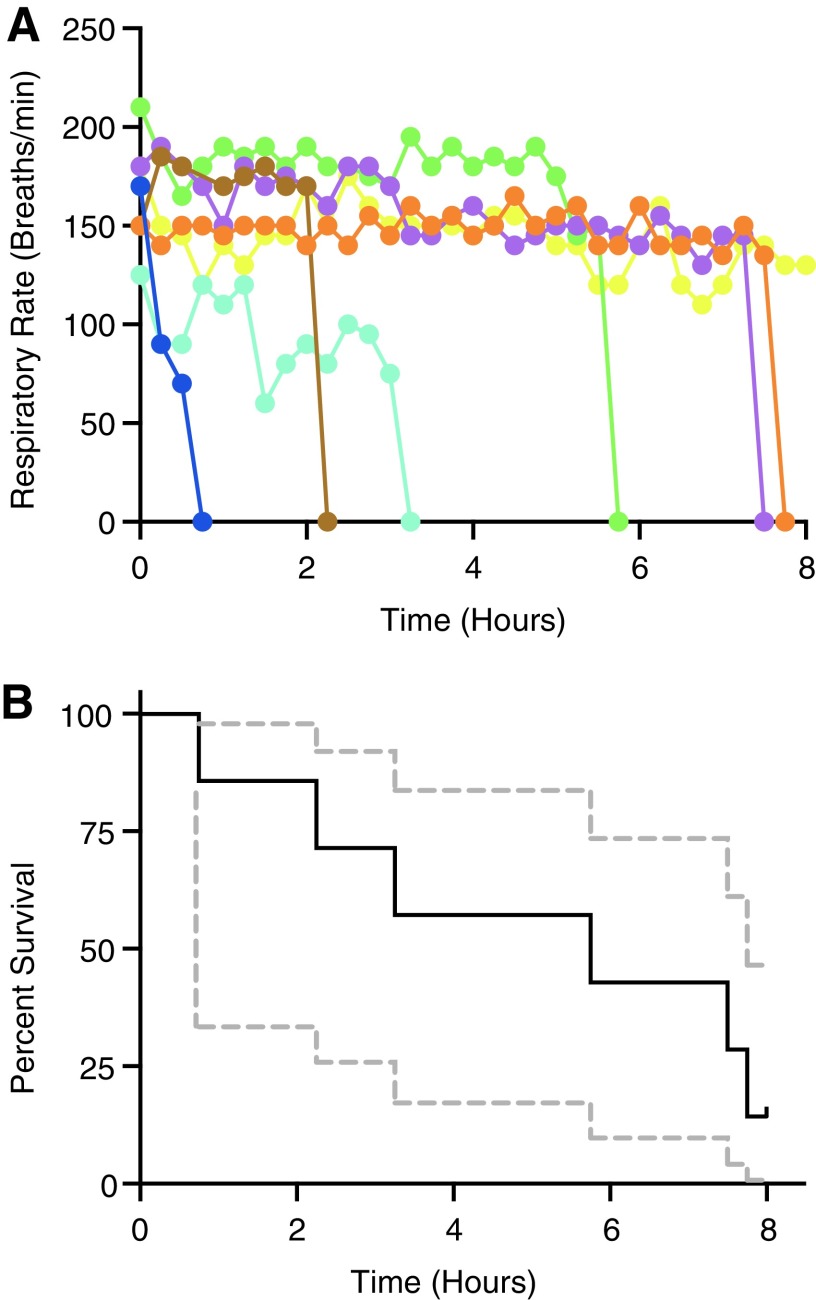
A systematic examination of the efficacy of benzocaine for long-term anesthesia in adult zebrafish has yet to be performed. We subjected adult fish to continuous administration of 0.0035% benzocaine and characterized anesthetic response over an 8-h period by monitoring respiratory rate. In seven individual trials, we observed high variability in time to respiratory arrest, with respiratory responses characterized by sudden and rapid drops in respiratory rate (Fig. 2A). Quantitatively, this variability was manifested as large 95% confidence intervals in Kaplan–Meier survival curves (Fig. 2B). We observed a 75% survival time (amount of time before which >75% of fish have not undergone respiratory arrest) of 2.25 h, a mean survival time (area under the survival curve) of 5.0 h, and an 8-h survival percentage of 14.3%. Cox regression analysis revealed that neither standard length (p = 0.10) or initial respiratory rate (p = 0.85) were significant predictors of survival. Measurements of initial water quality revealed that pH (7.5–7.8), water hardness (289 ± 11 mg/L), conductivity (609 ± 10 μS), and alkalinity (49 ± 3 mg/L) were similar across experiments. Further, they remained stable throughout the entire trial durations (<10% change within each trial). None of these parameters (pH: p = 0.13, hardness: p = 0.41, alkalinity: p = 0.52, conductivity: p = 0.10) were significant predictors of survival.
FIG. 2.
Respiratory rates and survival curves in continuously dosed zebrafish. (A) Respiratory rates over time for the seven individual fish. Note the abrupt termination of respiration for many of the zebrafish tested. (B) Kaplan–Meier survival curve for zebrafish continuously dosed with 0.0035% benzocaine solution. Ninety-five percent confidence intervals are indicated by dashed gray lines.
Intermittent administration of benzocaine and fresh water reveals differential kinetics of sedation and recovery
While continuous administration substantially increased anesthetic periods compared to that previously reported using MS-222 or MS-222/isoflurane cocktails,6 the low survival at 8 h (<15%) limited the practical utility of this regimen. To devise dynamic dosing schedules that increased 8-h survival, we developed a standardized test to characterize the kinetics of anesthetic induction and recovery. We performed six individual trials in which adult zebrafish were sedated in MS-222, perfused with fresh water until recovery to stage 1, perfused with benzocaine until stage 3 (time to sedation), and then reperfused with fresh water until stage 1 (time to recovery). Time to sedation (14.7 ± 1.3 min) was significantly (p < 0.01) greater than time to recovery (11.2 ± 0.6 min). Further, the coefficient of variation (CV), a normalized measure of dispersion, was higher for time to sedation (CV = 0.21) compared to time to recovery (CV = 0.13). This suggests that the period of time required for anesthetic induction was more variable compared to the period of time required for recovery.
Dynamic administration of benzocaine modulates anesthetic stages and prolongs survival
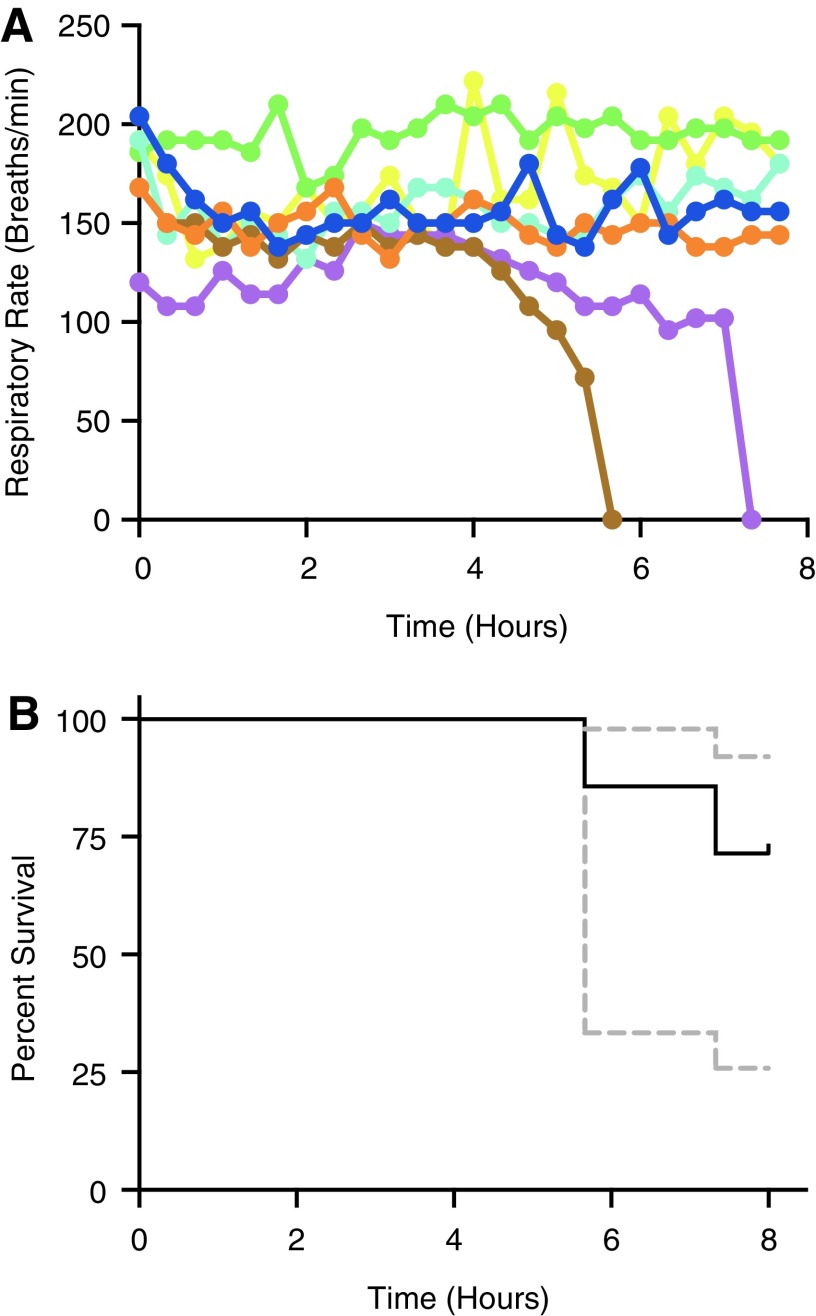
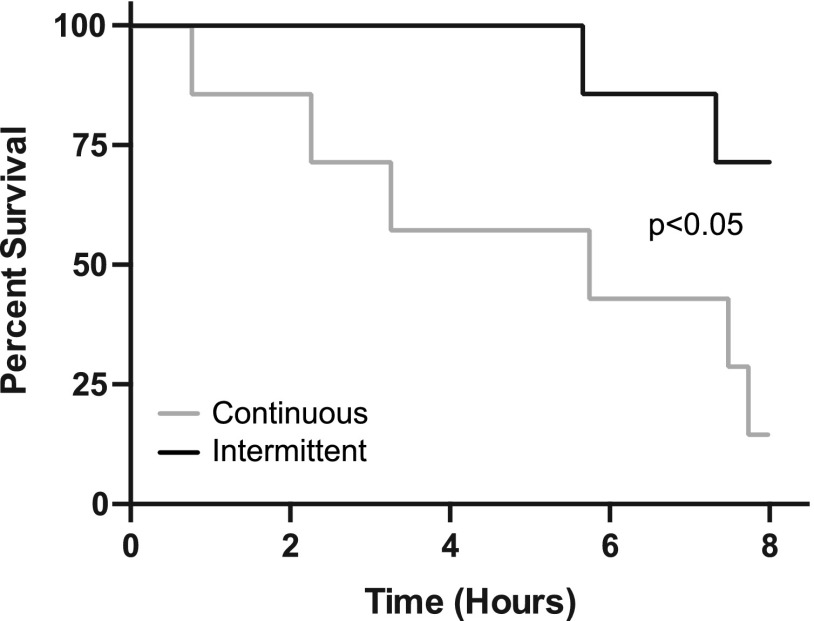
We postulated that an intermittent regimen consisting of ∼100% of the average time to sedation and ∼50% of the average time to recovery would maintain the fish within stage 3 anesthesia and prolong time to respiratory arrest compared to continuous administration. To test this, we subjected individual adult fish to intermittent administration of benzocaine (14.5 min) and fresh system water (5.5 min) and monitored respiratory rate over an 8-h period. For all fish, stage 3 was maintained for the majority of the trial, with most fish maintaining voluntary respiration through the entire trial period (Fig. 3A). Kaplan–Meier survival curves (Fig. 3B) were used to compute survival characteristics. Intermittent administration resulted in a 75% survival time of 7.3 h, a mean survival time of 7.6 h, and an 8-h survival percentage of 71.4%. Similar to our results under continuous administration, neither standard length (p = 0.73) or initial respiratory rate (p = 0.13) were significant predictors of survival. When using transgenic fluorescent reporter strains to assess image quality, we observed similar responses to wild-type animals, including 8-h survival under intermittent benzocaine administration. Cox regression analysis revealed that the difference in survival in fish administered continuous versus intermittent benzocaine (Fig. 4) was statistically significant (p < 0.05). When compared to continuous benzocaine administration, intermittent administration resulted in a 3.2-, 1.5-, and 5.0-fold increase in 75% survival time, mean survival time, and 8-h survival percentage, respectively.
FIG. 3.
Respiratory rates and survival curves in intermittently dosed zebrafish. (A) Respiratory rates over time for the seven individual fish. (B) Kaplan–Meier survival curve for zebrafish intermittently dosed with 0.0035% benzocaine solution. Ninety-five percent confidence intervals are indicated by dashed gray lines.
FIG. 4.
Comparison of survival curves in continuously and intermittently dosed zebrafish. Cox regression analysis revealed p < 0.05 for the difference in survival in fish administered continuous versus intermittent benzocaine.
Given that the majority (>70%) of animals survived to 8 h when administered benzocaine intermittently, we examined the potential for this regimen to extend anesthesia beyond an 8-h period. We subjected a single adult zebrafish to intermittent benzocaine administration and monitored respiratory rate over a 24-h period. Stage 3 anesthesia was maintained for the majority of the 24-h trial, with respiratory rates fluctuating between 140 and 168 breaths/min. These rates were comparable to those observed in fish that survived to 8 h in intermittent benzocaine trials (Table 2).
Table 2.
Respiratory Rate Comparison for Intermittent Imaging Trials
| 1 h | 3 h | 6 h | 12 h | 21 h | 24 h | |
|---|---|---|---|---|---|---|
| 8-h Dynamic flow | ||||||
| Mean RR | 154.8 | 162.0 | 168.8 | — | — | — |
| Range | 138–192 | 132–192 | 150–192 | — | — | — |
| 95% CI | 127–182 | 133–190 | 145–191 | — | — | — |
| 24-h Dynamic flow | ||||||
| Respiratory rate | 168 | 174 | 174 | 160 | 144 | 140 |
Respiratory rate (RR) presented in beats per minute, n = 5 for 8-h trials and n = 1 for the 24-h trial.
Discussion
The growing use of zebrafish to examine postembryonic physiologies has created a need for anesthetic regimens that facilitate long-term in vivo imaging in adult animals. Here, we show that continuous and intermittent administration of 0.0035% benzocaine in adult zebrafish enable mean times to respiratory arrest of 5.0 and 7.6 h, respectively. Immobilization was sufficient for long-term imaging; spontaneous movements were unusual, solitary in nature, and virtually absent beyond the first 1–2 h. Further, the effects of these movements on image quality were minimal as they were effectively suppressed by adhesion of the tail fin to the glass slide. No decreases in cell fluorescence were observed that would indicate cell death.
The exact mechanism of action for benzocaine has not been studied in zebrafish. However, due to its high structural similarity to MS-222, it likely acts similarly to MS-222 by inhibition of voltage-gated sodium channels.11,16–18 Studies comparing the efficacy of these anesthetics in amniotes show that when comparing the same exposure levels, benzocaine more quickly suppresses motor and neurological responses compared to MS-222, suggesting that benzocaine may possess increased potency at the cost of extended recovery times.15,17 Differences in anesthetic responses between the two compounds might arise from multiple factors including different pharmacodynamics at the sodium channel, different pharmacokinetics (e.g., differences in absorption and metabolism), and/or off-target effects. Benzocaine may alter the water quality to a different extent than MS-222 (e.g., different degrees of acidification). With a fish producing carbon dioxide under anesthesia, unless the final solution can sustain adequate buffering capacity, the resulting reduction in water pH can lead to acidosis, which will eventually lead to respiratory arrest and death due to metabolic derangements.25 In our system water quality parameters (temperature, pH, water hardness, conductivity, and alkalinity) were not good predictors of time to respiratory arrest. However, it is notable that unlike MS-222, benzocaine is pH neutral,18 minimizing the potential for acidosis. Finally, if the increased potency of benzocaine allows lower effective concentrations of the drug to be used (compare 0.0035% for benzocaine to 0.02% for MS-222), the effects of harmful metabolites and off-target binding might be reduced.
In addition to the use of different anesthetic agents, dosing schedules may also have a significant impact on survival during long-term anesthesia. Our dynamic perfusion system enables a wide spectrum of regimens to be administered to optimize for different compounds and fish sizes. All parts comprising our system are commercially available or able to be constructed using 3D printing. Further, our system is amenable to individually tailoring dosing intervals to extend survivability. For example, in our studies we noticed a fair amount of individual variation in times to sedation (and to a lesser degree, in times to recovery) when determining dosing intervals for the intermittent regimen. While we employed a generic dosing regimen (i.e., application of benzocaine for 100% of the time to sedation followed by application of system water for 50% of the time to recovery) by averaging across individuals, it is possible that regimens could be tailored to individual fishes. Specifically, the times to sedation and recovery for each fish may be measured at the onset of anesthesia, with the 100% time to sedation and 50% time to recovery implemented as a custom dosing interval individualized to each animal.
Several limitations of our study should be considered. First, given the effects of benzocaine on sodium channel activity, alternate anesthetic strategies may be required to examine physiologies whose actions are strongly coupled to this mechanism of action. Recent studies7,10 highlight a variety of active anesthetic compounds in adult zebrafish whose potential for long-term sedation have yet to be systematically explored. By integrating these compounds with dynamic administration (including intermittent administration of multiple compounds), our system provides new opportunities to optimize long-term anesthetic regimens with these compounds. Second, in mice, anesthetic susceptibility has been shown to depend on genetic background for select anesthetic agents.26 Future investigations are needed to determine the precise influence of genetic background on anesthetic efficacy, and may enable further optimization of anesthetic regimens.
In summary, our studies develop a novel dynamic system for long-term imaging in adult zebrafish, and demonstrate the potential for continuous and intermittent administration of benzocaine to enable prolonged anesthesia in adult animals. By establishing strategies that extend anesthetic durations in adult animals, our studies expand the experimental utility of adult zebrafish by facilitating real-time observation of biological events that proceed over the course of 1–24 h.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Joseph Sisneros from the University of Washington for assistance with benzocaine preparation. The authors would also like to acknowledge support from NIH Grant AR066061, UW Royalty Research Fund Grant A88052, and the University of Washington Department of Orthopedics and Sports Medicine.
Disclosure Statement
No competing financial interests exist.
References
- 1.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 2009;238:2975–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White R, Rose K, Zon L. Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 2013;13:624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson CJ, Kwon RY. Osteogenic programs during zebrafish fin regeneration. Bonekey Rep 2015;4:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poss KD. Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol 2007;18:36–45 [DOI] [PubMed] [Google Scholar]
- 5.Becker CG, Becker T. Adult zebrafish as a model for successful central nervous system regeneration. Restor Neurol Neurosci 2008;26:71–80 [PubMed] [Google Scholar]
- 6.Huang WC, Hsieh YS, Chen IH, Wang CH, Chang HW, Yang CC, et al. . Combined use of MS-222 (tricaine) and isoflurane extends anesthesia time and minimizes cardiac rhythm side effects in adult zebrafish. Zebrafish 2010;7:297–304 [DOI] [PubMed] [Google Scholar]
- 7.Collymore C, Tolwani A, Lieggi C, Rasmussen S. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 2014;53:198–203 [PMC free article] [PubMed] [Google Scholar]
- 8.Grush J, Noakes DL, Moccia RD. The efficacy of clove oil as an anesthetic for the zebrafish, Danio rerio (Hamilton). Zebrafish 2004;1:46–53 [DOI] [PubMed] [Google Scholar]
- 9.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). University of Oregon Press, Eugene, OR, 1993 [Google Scholar]
- 10.Readman GD, Owen SF, Murrell JC, Knowles TG. Do fish perceive anaesthetics as aversive? PLoS One 2013;8:e73773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frazier DT, Narahashi T. Tricaine (MS-222): effects on ionic conductances of squid axon membranes. Eur J Pharmacol 1975;33:313–317 [DOI] [PubMed] [Google Scholar]
- 12.Swinburne IA, Mosaliganti KR, Green AA, Megason SG. Improved long-term imaging of embryos with genetically encoded alpha-bungarotoxin. PLoS One 2015;10:e0134005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann A, Mickoleit M, Weber M, Huisken J. Multilayer mounting enables long-term imaging of zebrafish development in a light sheet microscope. Development 2012;139:3242–3247 [DOI] [PubMed] [Google Scholar]
- 15.Cakir Y, Strauch SM. Tricaine (MS-222) is a safe anesthetic compound compared to benzocaine and pentobarbital to induce anesthesia in leopard frogs (Rana pipiens). Pharmacol Rep 2005;57:467–474 [PubMed] [Google Scholar]
- 16.Neumcke B, Schwarz W, Stampfli R. Block of Na channels in the membrane of myelinated nerve by benzocaine. Pflugers Arch 1981;390:230–236 [DOI] [PubMed] [Google Scholar]
- 17.Ramlochansingh C, Branoner F, Chagnaud BP, Straka H. Efficacy of tricaine methanesulfonate (MS-222) as an anesthetic agent for blocking sensory-motor responses in Xenopus laevis tadpoles. PLoS One 2014;9:e101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross LG, Ross B. Anaesthetic and Sedative Techniques for Aquatic Animals, 3rd ed. Blackwell, Oxford; Ames, IA, 2008 [Google Scholar]
- 19.DeLaurier A, Eames BF, Blanco-Sanchez B, Peng G, He X, Swartz ME, et al. . Zebrafish sp7:EGFP: a transgenic for studying otic vesicle formation, skeletogenesis, and bone regeneration. Genesis 2010;48:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, Young RM, et al. . In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev Biol 2012;366:327–340 [DOI] [PubMed] [Google Scholar]
- 21.Laycock JD. Signs and stages of anaesthesia; a restatement. Anaesthesia 1953;8:15–20 [DOI] [PubMed] [Google Scholar]
- 22.Noga EJ. Fish Disease: Diagnosis and Treatment, 2nd ed. Wiley-Blackwell, Oxford, 2010 [Google Scholar]
- 23.Recidoro AM, Roof AC, Schmitt M, Worton LE, Petrie T, Strand N, et al. . Botulinum toxin induces muscle paralysis and inhibits bone regeneration in zebrafish. J Bone Miner Res 2014;29:2346–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly DW, Mawdesley-Thomas LE, Bucke D. Anaesthesia of fish. Vet Rec 1972;91:424–426 [DOI] [PubMed] [Google Scholar]
- 25.Neiffer DL, Stamper MA. Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. ILAR J 2009;50:343–360 [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Seo N, Kobayashi E. Genetic background differences between FVB and C57BL/6 mice affect hypnotic susceptibility to pentobarbital, ketamine and nitrous oxide, but not isoflurane. Acta Anaesthesiol Scand 2006;50:553–556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.