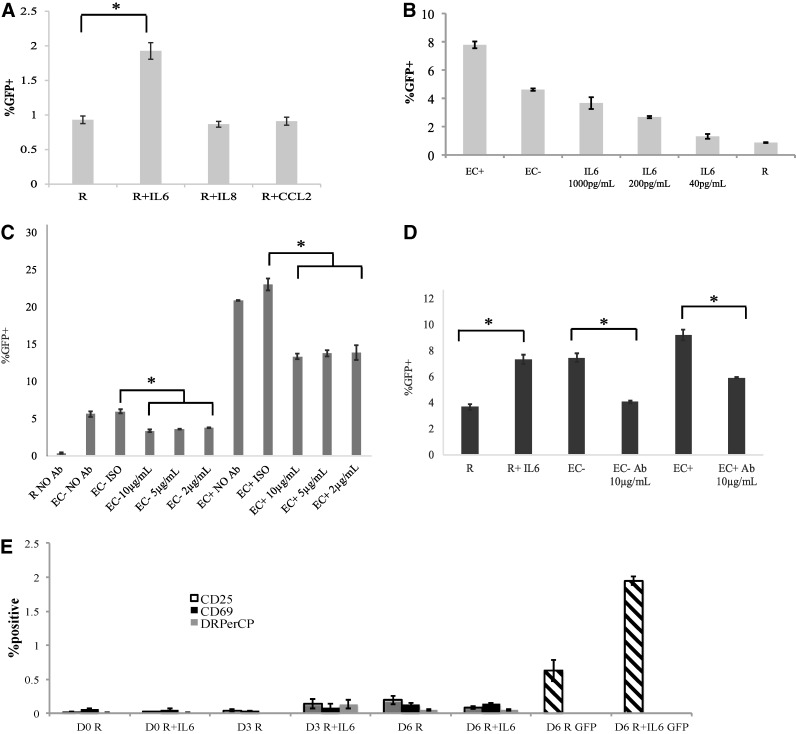
FIG. 5.
IL-6 stimulation increases productive infection in resting CD4+ T cells without T cell activation. (A) Three different cytokines, IL-6, IL-8, and CCL2, were added to resting CD4+ T cells 1 day before infection with a virus expressing GFP. Cytokines were replenished every other day after infection, and GFP-positive rates were measured on day 7 postinfection. (B) IL-6 concentration is positively associated with infection rates. Resting T cells were cultured with various concentrations of IL-6 1 day before infection with a reporter virus. The cytokine was replenished on days 1, 3, and 5 postinfection, and GFP was measured on day 6 postinfection. Resting T cells alone and resting T cells cultured with EC− or EC+ were used as controls. (C) EC stimulation is blocked by anti-IL-6 antibody. An anti-IL6 antibody (Ab) was added to EC+/− for approximately an hour before addition of resting T cells in direct contact at three concentrations (10, 5, and 2 μg/ml). After culturing for a day, the cells were infected with a virus expressing GFP. The antibodies were refreshed on days 1 and 3 postinfection, and GFP levels were measured on day 6 postinfection. (D) Similar to the experiment in (C), except that T cells were cultured in transwell with EC. Antibody level was at 10 μg/ml. Resting CD4+ T cells cultured alone or treated with IL-6 served as controls. (E) Lack of T cell activation by IL-6 stimulation. Resting CD4+ T cells were cocultured with IL-6 at 1 ng/ml for 1 day and then infected with a GFP reporter virus. CD25, CD69, and HLA-DR levels were measured on the day of infection (D0) and day 3 (D3) and 6 (D6) postinfection. The cytokine was replenished on days 1, 3, and 5 postinfection, and GFP was measured on day 6 postinfection (the right two bars). Samples were taken in triplicates, and means ± standard errors are plotted. Data shown are the representative of at least three independent experiments yielding similar results. *Student t-test; p < .05.