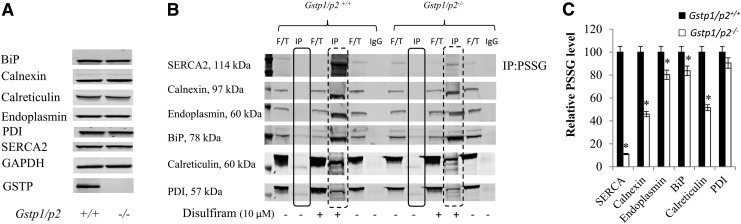
FIG. 2.
GSTP-mediated ER protein S-glutathionylation in Gstp1/p2+/+ and Gstp1/p2−/− mouse liver lysates. Liver tissues were freshly prepared from Gstp1/p2+/+ and Gstp1/p2−/− mice. (A) Baseline levels of calnexin, calreticulin, endoplasmin, SERCA, PDI, and BiP in Gstp1/p2+/+ and Gstp1/p2−/− samples. (B) One milligram of liver lysates untreated or treated with disulfiram (10 μM, for 30 min at 37°C) was used for IP with protein A/G-agarose beads using mouse monoclonal anti-PSSG antibodies. Samples, including IP with anti-PSSG, IP with mouse IgG, and F/T after precipitation by A/G-agarose beads, were analyzed by subsequent nonreducing SDS-PAGE and blotted with anti-calnexin, calreticulin, endoplasmin, SERCA, PDI, or BiP antibodies. Solid squares show ER-resident protein levels after IP with anti-PSSG in untreated samples, and dashed squares show ER-resident protein levels after IP with anti-PSSG in disulfiram-treated samples. (C) Quantification of fluorescent intensities of the bands in dashed squares shown in (B). *Represents significant difference between Gstp1/p2+/+ and Gstp1/p2−/− mice. Data are representative blots from three independent experiments. F/T, flow-through; IgG, immunoglobulin G; IP, immunoprecipitation; PSSG, Protein S-glutathionylation; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.