Abstract
β-toxin is an important virulence factor of Staphylococcus aureus, contributing to colonization and development of disease.1–3 This cytotoxin has two distinct mechanisms of action: sphingomyelinase activity and DNA biofilm ligase activity. However, the distinct mechanism which is important for its role in infective endocarditis is unknown. We characterized the active site of β-toxin DNA biofilm ligase activity by examining deficiencies in site-directed mutants through in vitro DNA precipitation and biofilm formation assays. Possible conformational changes in mutant structure compared to wild type toxin were assessed via trypsin digestion analysis, retention of sphingomyelinase activity, and predicted structures based on the native toxin structure. We addressed the contribution of each mechanism of action in producing infective endocarditis in vivo in a rabbit model of infective endocarditis and sepsis. The H289N β-toxin S. aureus mutant, lacking sphingomyelinase activity, was decreased in lethality and infective endocarditis vegetation formation compared to wild type protein. β-toxin mutants disrupted in biofilm ligase activity did not decrease S. aureus lethality, but were deficient in infective endocarditis vegetation formation compared to wild type protein. Our study begins to characterize the DNA biofilm ligase active site of β-toxin and suggests β-toxin functions importantly in infective endocarditis through both of its mechanisms of action.
Keywords: Staphylococcus aureus, cytotoxin, biofilm, sphingomyelinase
Infective Endocarditis (IE) is a life threatening infection of native/prosthetic valves and the lining of the heart. IE is characterized by the formation of vegetations, “cauliflower-like” structures composed of bacteria and host factors.4–9 Each year there are as many as 100,000 cases of IE in the United States10. IE results in local and distal complications8. Often, pieces of septic vegetations detach leading to systemic embolization and metastatic infections resulting in strokes, infarcts, and/or abscesses that can cause persistent bacteremia, organ failure, and death in up to 66% of patients.9, 11, 12 Staphylococcus aureus is the most commonly identified pathogen in patients with healthcare-associated IE, accounting for 40% of cases, and is the leading cause of community-associated IE in the developed world.12
Thirty to forty percent of the population is asymptomatically colonized with S. aureus. These carriers are more likely to develop S. aureus infections. S. aureus produces a variety of cell surface and secreted virulence factors which are important to its ability to cause disease such as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), cytotoxins, and superantigens (SAgs). Roughly 40% of S. aureus isolates from IE patients belong to the USA 200 clonal group, all of which produce the cytotoxin β-toxin.12, 13 Additionally, USA100 and USA400 clonal groups of S. aureus, are also capable of causing IE in part dependent on production of β-toxin1, 12, 13 through in vivo of excision of β-toxin-gene-inactivating bacteriophages.1 Having S. aureus bacteremia is a critical factor for developing S. aureus IE. In the United States and Europe, S. aureus is the second most commonly isolated pathogen from bloodstream infections.13 Thirteen percent of septicemia isolates produce β-toxin, and β-toxin has been demonstrated to aid in S. aureus bloodstream survival.14, 15 However, the percentage of positive strains for β-toxin is likely to be higher given the loss of β-toxin-inactivating bacteriophages in vivo.1
Huseby et al. have shown β-toxin is critical for causing IE in a rabbit model that strongly resembles human disease.2 The S. aureus strain COL (hlb+) forms large vegetations of about 220 mg, in comparison to a COL hlb− strain forming vegetations of only about 2.5 mg.2 Similarly, in the MW2 strain, a β-toxin+ variant produces larger vegetations than MW2 wild type in the same rabbit model.1 Furthermore, the frequency of β-toxin producing variants increases within vegetations during infection with wild type MW2, thus favoring the production of β-toxin through bacteriophage excision.1 There is also evidence β-toxin contributes to S. aureus colonization. Hiramatsu et al. demonstrated that when the β-toxin gene is intact, the strain NCTC 8325-4 persists longer in the nasal cavity than when the β-toxin gene isdisrupted.3
β-toxin has a molecular mass of 35 kDa, a basic pI (>10.0), is a member of the DNase I superfamily, and is a neutral sphingomyelinase (SMase).1, 14 Unlike other cytotoxins, β-toxin does not form pores in plasma cell membranes, but instead hydrolyses the plasma membrane lipid, sphingomyelin, into ceramide and phosphorylcholine.15 The SMase activity of β-toxin is characterized by its ability to lyse sheep erythrocytes. Orthologs of S. aureus β-toxin can be found in S. schleiferi, S. epidermidis, and Leptospira interrogans; there is high sequence similarity of the S. aureus β-toxin gene and its structure to SMases in Listeria ivanovii, and Bacillus cereus.14
β-toxin also has DNA biofilm ligase activity. The active site for the DNA biofilm ligase activity is uncharacterized but is distinct from the SMase active site. The DNA biofilm ligase property of β-toxin is defined by its ability to cross-link to itself in the presence of DNA.2 β-toxin covalently oligomerizes and precipitates in the presence of exogenous DNA, such as that found in biofilms. An hlb− strain of S. aureus shows reduced adherence in static assays and reduced biofilm growth in flow cell assays compared to an isogenic hlb+strain.2
While a role for β-toxin has been demonstrated in IE, the mechanism of action that it uses to cause its effects has not been elucidated. Considering the active site of the DNA biofilm ligase activity of β-toxin is unknown, we first began to characterize this active site through the construction of site-directed mutants. We determined mutants T149A, H162A, and D163A were deficient in a DNA precipitation assay. A biofilm formation assay was conducted to test further for disruption of the active site, in which mutants T149A and H162A were unable to form biofilms comparable to wild type β-toxin. Mutants T149A, H162A, and D163A were assessed for observable conformational changes by testing for retention of SMase activity, predicted structures, and comparable trypsin resistance as wild-type toxin. All mutants were able to lyse sheep erythrocytes and appeared to maintain native toxin overall conformation. Mutants H162A and D163A had similar stabilities as wild-type protein to heat and trypsin treatment.
We subsequently determined the contribution of each mechanism of action, SMase versus DNA biofilm ligase activity, of β-toxin in causing IE and sepsis in a rabbit model. The contribution of each activity to lethality and vegetation formation was determined. The SMase activity of β-toxin increased lethality and vegetation size. The DNA biofilm ligase activity of β-toxin did not affect lethality but increased vegetation size.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
COL, an early MRSA strain from the 1960’s and RN450, a non-mutagenized version of S. aureus RN4220, both naturally encoding for β-toxin, were utilized from lyophilized stocks of low passage maintained in the laboratory. S. aureus strains were grown in Todd Hewitt (TH) broth (Becton Dickinson, Sparks, MD), and E. coli was grown in lysogeny broth (LB) Miller formulation, both at 37 °C with aeration (225 RPM). Plasmids were maintained using erythromycin (20 μg/ml) or carbenicillin (100 μg/ml).
Construction of RN450 Δhlb nuc-and COL hlb-
COL hlb- was previously isolated from the original COL stock through disruption of the hlb gene by insertion of a βC bacteriophage.2 RN450Δhlb nuc- was generated by making in-frame deletions of hlb. Briefly, PCR products were amplified with primers sets hlb-upstream.F (5′-atcctagaattcgcagacgcttcatta-3′)/hlb-upstream.R (5′-GCTAGCACGCGTGTTTTTTTCACCATC-3′), and hlb-downstream.F (5′-cgatcgtgcgcagcctatagtaaata-3′)/hlb-downstream.R (5′ATCCTACCCGGGGGTCTGGTGAAAAC-3′). Amplifications were spliced together by overlapping PCR with hlb-upstream.F/hlb-downstream.R and inserted into pJB38. The resulting plasmid was electroporated into RN4220, from which a lysogen of bacteriophage ф11 containing the plasmid was made, the lysogen was used to introduce the plasmid into RN450 by transduction. The deletion was introduced via allelic exchange and verified by PCR, and loss of sheep blood hemolysis. The nuclease gene, nuc, was disrupted in this strain by using the Targetron Gene Knockout System (Sigma, TA0100) nuc specific insertion plasmid, and transduced into RN450Δhlb as previously described and confirmed by PCR.16
Expression of β-toxin in RN450 Δhlbnuc -and COL hlb-
hlb was amplified from RN4220 using primer set kpnI.btoxin.2F (5′-GCATCTTTATACTCAAAAAACATTT-3′), and ecorI.btoxin.R (5′-AAAGAATTC GTTTGAGTGTAGAGTAAAGACTTG-3′). The PCR product was ligated into plasmid pCE104, and transformed into E. coli DH5α. The resulting plasmid was electroporated into S. aureus RN4220, from which a lysogen of bacteriophage ф11 containing the plasmid was made. This lysogen was used to move the plasmid into RN450 Δhlb nuc- and COL hlb-. Expression was characterized by sheep blood hemolysis. Mutations T149A, T151A, C158A, H162A, D163A, and H289N were made using QuikChange (Stratagene) site-directed mutagenesis and moved into RN450 Δhlb nuc-and COL hlb- as described for wild type β-toxin.
DNA Precipitation Assay
DNA precipitation assays were performed by Huseby et al..2 Briefly, wild-type and β-toxin mutants were combined with 12 μg of Qiagen maxi prep purified pCE104 plasmid DNA in molecular grade H2O and 1.2 μg of ethidium bromide, incubated at 37 °C for 30 minutes, incubated on ice for 10 minutes, and centrifuged for 10 minutes at 10,000×g. DNA precipitation was visualized by photography.
Biofilm Formation Assay
Cultures were grown overnight in tryptic soy broth (TSB) (MP Biomedicals, LLC) at 37 °C with aeration. These cultures were diluted to 1.5×108 colony-forming units (CFUs)/ml. These were further diluted 1:100 in TSB supplemented with 2% (wt/vol) glucose and 2% (wt/vol) NaCl. Approximately 200 μl of these final dilutions was transferred to a 96-well plate (Costar, Corning Inc., NY, USA), previously coated with 20% human plasma diluted in bicarbonate/carbonate buffer. The cultures were incubated at 37 °C under static conditions overnight. Then, 150 μl of culture was removed from each well and replaced with 150 μl fresh TSB supplemented with 2% glucose and 2% NaCl, and then incubated under the same conditions overnight again. The absorbance was measured on a plate reader at 562 nm wavelength to measure total growth. The cultures were removed from the wells, and each was washed three times with 200 μl PBS. The biofilm was fixed by adding 100 μl 100% ethanol to each well. The plates were then air dried. Each biofilm was stained with 100 μl of 0.1% crystal violet in deionized water at room temperature for 10 minutes. The crystal violet was removed, and each well was washed three times with 200 μl PBS. To elute, 100 μl of 80%ethanol/20% acetone was added per well and incubated at room temperature for 20 minutes. Each well was thoroughly mixed and diluted 1:10 in 80% ethanol/20% acetone. The absorbance of the dilution was measured on a plate reader at 562nm wavelength.
Protein Production
β-toxin used for DNA precipitation assays was collected from RN450 Δhlb nuc- containing plasmid pCE104 and expressing hlb. Overnight cultures were treated with 4 volumes of 100% EtOH to precipitate β-toxin. The precipitate was dried and resuspended in deionized water to 1/50th the original culture volume. The diameter of hemolysis was measured from each of the concentrates at various amounts on microscope slides coated with 1% agarose (Sigma Aldrich, St. Louis, MO) containing sheep erythrocytes, using NIH image J.1, 17 The β-toxin in the concentrates was then quantified by comparing lysis diameters to a standard curve made from lysis diameters of purified recombinant wild type β-toxin at known concentrations.
β-toxin was cloned into pTrcHis TOPO vector (Invitrogen Life Technologies, Grand Island, NY) using primer set btoxin.3F (5′-GAATCTAAGAAAGATGATACTGATTTG-3′)/btoxin.2R (5′-CTATTTACTATAGGCTTTGATTGGG-3′) and expressed in E. coli DH5α. E. coli expressing the vector was grown in 1 L terrific broth (TB) with 100 μg/ml carbenicillin at 37 °C, shaken at 225 rpm to an OD600 of 0.5. Protein production was then induced with 1 ml of 1 M isopropyl-β-D-thiogalactopyranoside (IPTG), followed by incubation at 30 °C for 18 hours. The cells were lysed to release protein via passage through a microfluidizer high shear fluid processor and batch purified using under native conditions with using cobalt-linked agarose resin (Sigma, St. Louis, MO). The eluted protein was dialyzed in 12–14kD molecular weight cutoff dialysis membrane against 1 L of PBS for 24 hours. Biofilm ligase mutants H162A and D163A were purified by the methods used for wild type β-toxin. With staphylococcal enterotoxin B as a standard, protein concentrations were determined by the Bradford assay(BioRad).
The structure of β-toxin was previously determined. Cartoon presentations of the structure of β-toxin was constructed using The PyMOL Molecular Graphics System, Version 1.8.0 Schrödinger, LLC. The location of mutated amino acid residues were mapped onto the structure.
Trypsin Treatment of β-toxin
Recombinant purified β-toxin (100 μg/ml) was incubated at 37 °C with trypsin (50 μg/ml in 10 mmol/L Tris-HCl, pH 8.0) to a final volume of 500 μl for 0, 0.5, 1–6, 8, 12, and 24 hours. After incubation for the desired time, 50 μl of the 500 μl was removed and inactivated by incubation at 95 °C for 5 minutes followed by immediate storage at −20 °C. Each 50 μl each time point was mixed with 50 μl of SDS-PAGE sample buffer, and boiled for 5 minutes prior to assessment by SDS-PAGE.
Rabbit Model of Infective Endocarditis and Sepsis
All animal experiments were performed according to guidelines and protocols approved by the University of Iowa Institutional Animal Care and Use Committee (Protocol 1106140 which was replaced by current protocol 4071100). The experiment was performed as previously described.18 Briefly, New Zealand white rabbits, 2–3 kg of both sexes, were anesthetized with ketamine (25mg/kg) and xylazine (25mg/kg) (Phoenix Pharmaceuticals, Burlingame, CA). A catheter was inserted into the left carotid artery of each animal until the aortic valve was reached and left in place for 2 hours to induce damage, removed, and incision site closed. Subsequently, bacteria were injected through the marginal ear veins. The experiments were allowed to proceed up to 4 days. Rabbits were treated with erythromycin to maintain the plasmid in the strains. Heart vegetations were dissected, weighed, homogenized, and plated to enumerate bacterial CFUs/total vegetations within each heart. Statistical significance in survival experiments was determined using the Log-rank, Mantel-Cox test (GraphPad Prism Software). Significance across means was carried out using the Mann-Whitney test (GraphPad Prism Software).
RESULTS
Site-Directed Mutagenesis Disrupts β-Toxin Oligomerization in a DNA Precipitation Assay
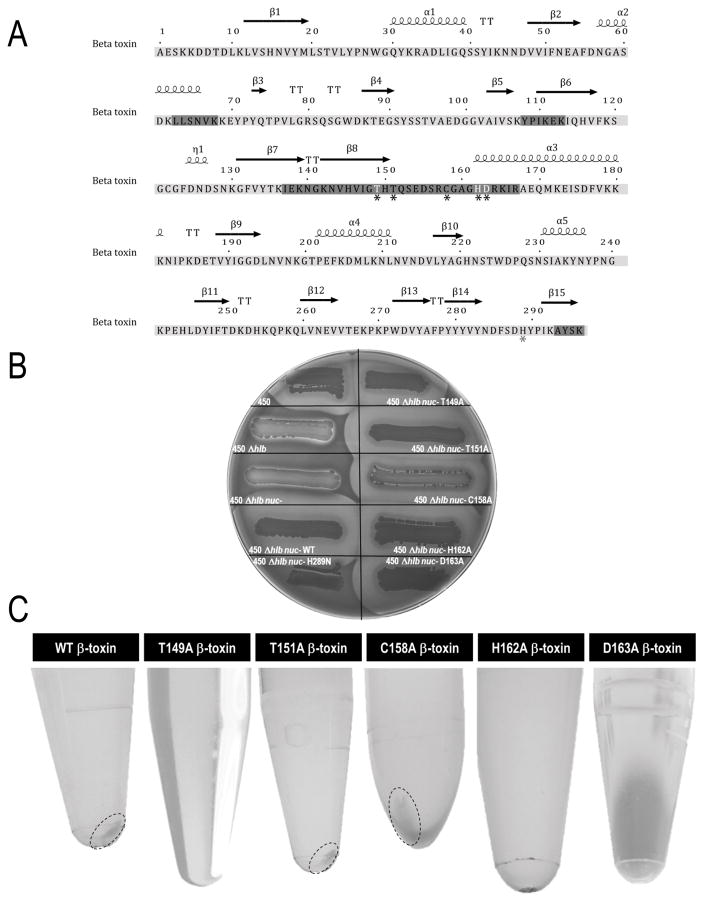
To begin to characterize the DNA biofilm ligase active site, mutagenesis was concentrated in the Asn143-Arg164 amino acid region of β-toxin as suggested in a prior study by Huseby et al.2 (see Fig 1a). Site directed mutants T149A, T151A, C158A, H162A, and D163A were constructed and expressed in S. aureus RN450Δhlb nuc-. β-toxin was deleted, and nuclease was disrupted in the RN450 laboratory strain to allow for testing of mutants for deficiencies in the DNA precipitation assay without the interference of native β-toxin or DNA degradation due to nuclease. Mutations did not affect the SMase active site, as seen by retention of sheep erythrocyte lysis (Fig 1b). Protein concentrates were obtained from RN450Δhlb nuc- strains expressing wild type, T149A, T151A, C158A, H162A, or D163A β-toxin and β-toxin quantified. Proteins were tested in the DNA precipitation assay with β-toxin protein concentrations ranging from 35 μg to 200 μg. Wild type β-toxin formed a precipitate beginning at 60 μg of protein. Mutant T151A was similarly able to form a precipitate beginning at 50 μg, and mutant C158A beginning at 60 μg of β-toxin. Mutants T149A, H162A, and D163A were not able to form precipitates at up to 200 μg of β-toxin (Fig 1c).
Figure 1.
Mutagenesis does not affect SMase activity but inhibits the ability of β-toxin to oligomerize. (a) Amino acid sequence of β-toxin. Highlighted areas are regions predicted to be biologically important by Huseby et al.2, 14 Black asterisks denote residues mutated, those in white indicate changes that disrupted the DNA biofilm ligase activity. Gray asterisk indicates residue mutated to disrupt SMase activity. (b) All DNA biofilm ligase mutants retain ability to lyse sheep blood erythrocytes similar to wild type as seen by zones of lysis surrounding growth on sheep blood agar plates. (c) DNA precipitation assays performed with β-toxin recovered from expression in S. aureus RN450Δhlb nuc-. Mutants T149a, H162A, and D163A were unable to form precipitates at up to 200 μg of β-toxin. Wild type and mutant T151A formed a precipitate beginning at 50 μg of protein, and C158A at 60 μg.
Mutants Unable to Oligomerize in the DNA Precipitation Assay are Deficient in Biofilm Formation
Previous data demonstrate that a decrease in biofilm formation was seen in S. aureus strain COL following hlb disruption, and therefore we investigated the ability of mutants, defective in the DNA precipitation assay, to form biofilms in vitro in this strain.2 β-toxin wild type and mutants T149A, H162A, and D163A were expressed in COL hlb- to test for deficiencies in biofilm formation without interference from endogenous β-toxin. Optical density of cultures in the 96-well plates was measured to rule out differences in growth between the strains that could account for deficiencies in biofilm formation; no growth differences were observed (Fig S1a). COL hlb- was significantly reduced in its ability to form biofilms compared to COL or the complemented strain, COL hlb- + hlb. Wild type β-toxin complemented COL hlb- made biofilms comparable to COL expressing native β-toxin. COL hlb- complemented with β-toxin T149A or H162A formed significantly less biofilms than COL hlb- + hlb at levels similar to COL hlb-. COL complementation with β-toxin D163A was still able to form biofilms similar to COL hlb- + hlb (Fig 2, S1b).
Figure 2.
DNA precipitation assay-deficient mutants are poor at forming a biofilms in a static biofilm assay in the S. aureus COL strain. Mutants T149A and H162A made significantly less biofilms than wild type. Mutant D163A still made biofilms at similar levels to wild type.
Protein Analysis Detect Minimal or No Major Conformational Changes in Mutants
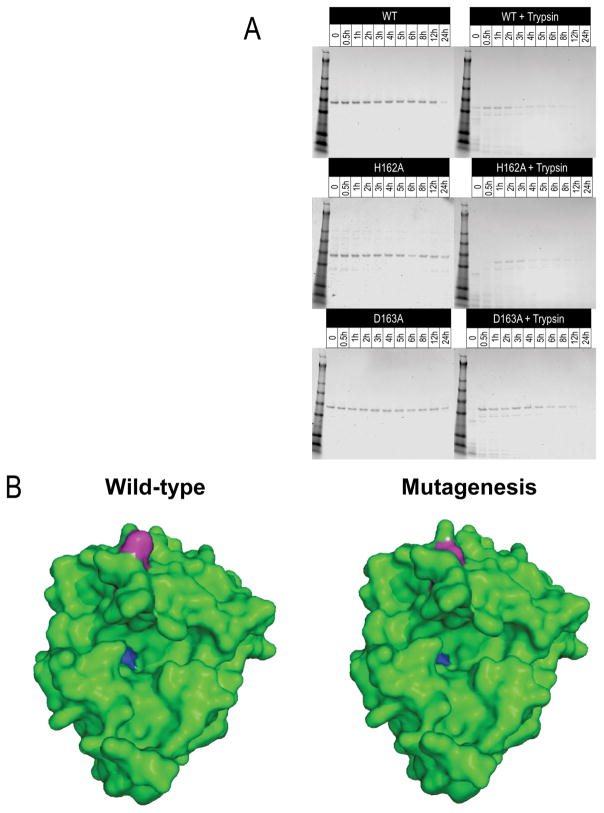
The mutants were tested to determine if deficiencies in oligomerization and biofilm formation were the result of major conformational changes in the proteins caused by the mutagenesis. The β-toxin mutants T149A, T151A, C158A, H162A, and D163A retain their SMase activity as seen by sheep blood hemolysis, and are thus maintain correct conformation for this activity when expressed in S. aureus RN450Δhlb nuc- (Fig 1b). Furthermore, recombinant His6-tagged wild type, H162A and D163A β-toxin proteins were purified and tested for their stability to heat and trypsin digestion. All purified proteins are readily detectable as a band at ~46 kDa corresponding to His6-tagged β-toxin after treatment at 37 °C for up to 24 hours with a minimal decrease in quantity, as indicated by loss of band intensity, at 24 hours (Fig. 3a). Following trypsin treatment, both mutants were susceptible to proteolysis at rates and displayed patterns similar to wild type β-toxin. SDS-PAGE showed a shift of the prominent band to a smaller size and several smaller bands immediately following treatment, further fading with increased duration of treatment. Bands are visible at 12 hours but no longer detectable at 24 hours (Fig 3a). We located the mutagenized residues onto the native β-toxin structure as previously determined by Huseby et al. (Fig 3b).14 All residues were determined to be surface amino acids. Our prior experience from mutagenesis and structural analyses of large numbers of staphylococcal superantigens indicate that mutagenized surface amino acids, important in activities, do not alter the overall structure of the proteins, but instead alter only the amino acid side chains.
Figure 3.
Mutagenesis does not alter β-toxin protein structure. (a) Trypsin digest analysis of mutants is comparable to wild type β-toxin proteolysis from 0-24 hours. Wild type and mutants H162 and D163 were stable at 37 °C for up to 24 hours, and had similar trypsin degradation rates and patterns. (b) Three-dimensional structure of β-toxin wild type compared to modeled mutants in DNA biofilm ligase and SMase activities. The T149, H162, and D163 DNA biofilm ligase inactivating residues are in magenta. The H289 SMase inactivating residue in blue. Residues H162 and D163 are in the same region toward the top of the protein, while T149 is near residue H289, obscured but partially on the surface. Amino acid substitutions at these residues do not appear to significantly change the predicted protein structure compared to wild type.
Sphingomyelinase Activity and Not DNA Biofilm Ligase Activity of β-toxin contributes to Lethality in a Rabbit Model of IE and Sepsis
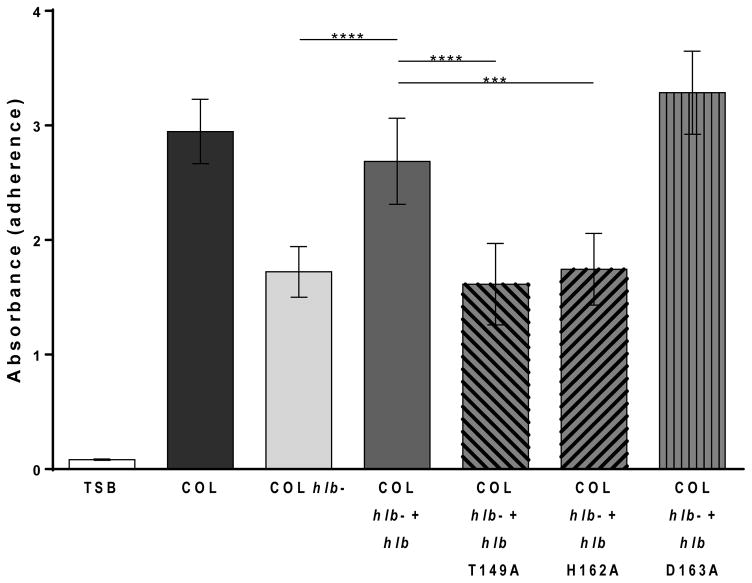
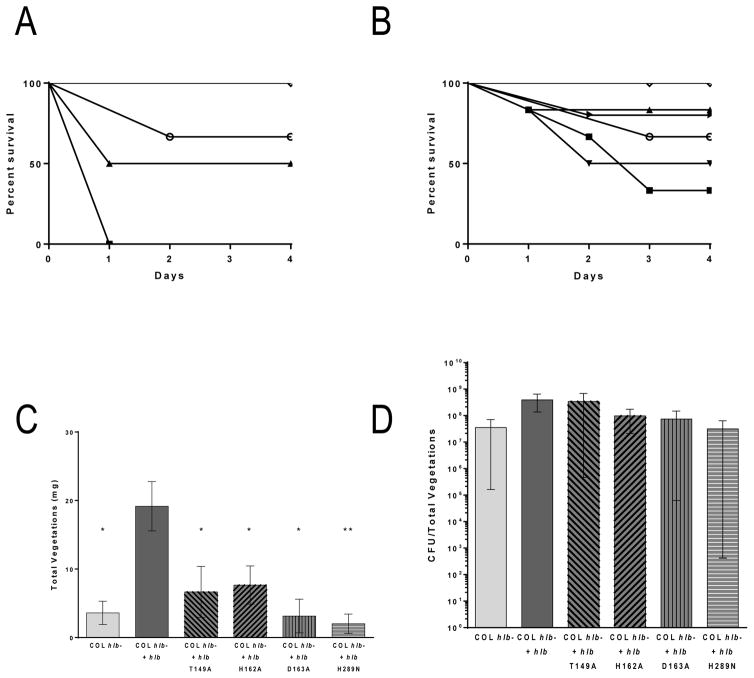
S. aureus COL hlb- expressing wild type β-toxin, a DNA biofilm ligase mutant, or a previously characterized SMase mutant, were tested in a rabbit model of IE and sepsis.2, 14 β-toxin production in tested strains was confirmed and quantified in sheep red blood cell hemolysis assays (Fig S2a). All strains made β-toxin at levels within 2-fold that of endogenous β-toxin produced by COL. Previous studies determined the lethal dose 50% endpoint (LD50) of COL to be 2×109 CFUs. To account for the increased β-toxin production in the plasmid carrying strains, a lower dose was injected into the rabbits intravenously at 5×108-1×109 CFUs and experiment allowed to progress for up to 4 days to assess lethality. Rabbits with COL hlb- plus hlb had a statistically significant increase in lethality compared to those with the strain lacking β-toxin in the vector, COL hlb-. Rabbits injected with the DNA biofilm ligase mutants had the same level of lethality as those rabbits injected with the wild type β-toxin carrying strain. However, rabbits infected with COL hlb- complemented with the β-toxin SMase mutant H289N were significantly decreased in lethality compared to rabbits infected with COL hlb- plus hlb, with the same level of lethality as COL hlb- (Fig 4a).
Figure 4.
β-toxin contributes to lethality and vegetation formation in a rabbit model of IE. (a) At an inoculum of 5×108-1×109 CFUs, rabbits infected with COL hlb- + hlb (■), COL hlb- + hlb T149A (▲), COL hlb- + hlb H162A (▼), or COL hlb- + hlb D163A (▶), died rapidly. Mostly, rabbits infected with COL hlb- (○) and COL hlb- + hlb H289N (◇) survived until the end of the experiment. (b) At an inoculum of 5×107, there was no difference in survival between the strains. (c) COL hlb- + hlb T149A, COL hlb- + hlb H162A, COL hlb- + hlb D163A, COL hlb- + hlb H289N, or COL hlb- infected rabbits produced little to no vegetations compared to COL hlb- + hlb infected rabbits at the lower inoculum. Asterisks denote statistical significance compared to COL hlb- + hlb (d) Vegetations from all rabbits infected at the lower inoculum had similar CFUs.
Sphingomyelinase and Biofilm DNA Ligase Activities of β-toxin Contribute to Vegetation Formation but Not Bacterial Burden within the Vegetations in a Rabbit Model of IE and Sepsis
To determine the contribution of the SMase and the DNA biofilm ligase activity to vegetation formation, the COL hlb- strains complemented with wild type β-toxin, DNA biofilm ligase mutants, or the SMase mutant were tested in the rabbit model of IE and sepsis. The rabbits were injected with 5×107 CFU, a dose that results in similar rabbit survival across strains and allows vegetation size to be compared within similar time frames (Fig 4b). Total vegetation size per rabbit was significantly larger in those infected with COL hlb- plus hlb, compared to those infected with the strain lacking β-toxin in the vector, COL hlb- (averaging 19.2 mg versus 3.6 mg, respectively). COL hlb- strains expressing DNA biofilm ligase mutants, T149A, H162A, or D163A were deficient in the ability to form vegetations compared to the wild type averaging 6.7 mg, 7.7 mg, and 3.1 mg respectively. Interestingly, COL hlb- expressing the SMase mutant H289N was also deficient in vegetation formation averaging 2.0 mg, which is similar in size to COL hlb- (Fig 4c, S2b). Despite the significant decrease in vegetation size seen in the DNA biofilm ligase and the SMase mutants, and the β-toxin deficient strain, there was no difference in CFUs recovered from dissected vegetations (Fig 4d), suggesting that the primary role of β-toxin in S. aureus IE is to promote accumulation of host factors and vegetation growth on infected valves.
DISCUSSION
S. aureus IE is a debilitating and rapidly progressing infection. Early diagnosis and treatment are difficult, and as such it results in death in up to 66% of cases.11 IE can cause destruction of the heart valves, complete obstruction of blood flow leading to congestive heart failure, as well as causing infection in the lungs, kidneys, spleen, and brain.8 Previous research has shown a role for β-toxin in IE in a rabbit model. However, the specific mechanism of action the cytotoxin uses for its effects in IE is unknown.
In this study we begin to characterize amino acid requirements for the DNA biofilm ligase activity of β-toxin. We further examine the role each mechanism of action of β-toxin has in causing IE and lethal sepsis. Our data show residues T149, H162, and D163 are essential in the active site of the DNA biofilm ligase activity. Using the dual IE and sepsis rabbit model, we demonstrate SMase activity contributes to the lethality β-toxin causes. Additionally, our data clearly show both the SMase activity and DNA biofilm ligase activity of β-toxin promote formation of the vegetations pathognomonic of the disease. Vegetation formation is necessary for IE progression, this suggests β-toxin plays a critical role in the disease via both of its mechanisms of action.
Mutants T149A, H162A, and D163A were unable to oligomerize in the presence of DNA in a DNA precipitation assay, indicating these mutants are disrupted in the overall DNA biofilm ligase active site necessary for this function. Evidence showing that mutants T149A and H162A are deficient in a biofilm formation assay compared to wild type β-toxin confirms that the DNA biofilm ligase active site is disrupted in these mutants. The similar proteolytic rates and profiles of β-toxin mutants H162A and D163A compared to wild type in a trypsin digest analysis suggests that the single amino acid substitutions did not significantly change the protein conformation and stability. Furthermore, the predicted protein structures of mutants are likely to remain unaltered compared to wild type toxin. An intact SMase active site as tested in the sheep blood erythrocyte lysis assay also supports this conclusion. Future studies to characterize the biofilm active site more thoroughly are being directed to co-crystallization and structure determination of β-toxin in the presence of DNA.
To determine how the SMase and DNA biofilm ligase activities contribute to the role of β-toxin in IE, disruption mutants of each were tested in the rabbit model of IE and sepsis. We used a high bacterial inoculum to determine if β-toxin affects lethality and to examine which mechanism it uses to do so. We found all but one of the rabbits infected with wild type or biofilm ligase mutants succumbed on the first day following infection, suggesting β-toxin enhances lethality to which the DNA biofilm ligase activity does not contribute. In contrast, all but one rabbit infected with the SMase mutant or lacking β-toxin survived to the end of the experiment suggesting the SMase activity may be responsible for the increase in lethality associated with β-toxin production. How the SMase activity of β-toxin increases lethality is yet to be characterized. Rabbits infected with wild type or DNA biofilm ligase mutants, which have only the SMase activity intact, die too quickly to form vegetations at this inoculum, and thus vegetation related complications are not plausible reasons for their deaths. Sphingomyelin is found at varying degrees, depending on cell type, in cell membranes throughout the host. The SMase activity of β-toxin could therefore affect many cell types during infection, leading to lethality.
Since previous studies have shown β-toxin increases vegetation size, we used a lower inoculum to examine which mechanism promotes vegetation growth. We observed that both the DNA biofilm ligase mutants and the SMase mutant formed significantly smaller vegetations, on average 5.8 mg and 2 mg respectively, compared to wild type which formed vegetations averaging 19.2 mg. Both mutants had vegetations similar in size to the β-toxin deficient strain, suggesting that while both activities increase vegetation size in IE, their contributions are not additive.
The SMase activity of β-toxin may increase vegetation size, but how it does so is not clear. It is possible β-toxin helps initiate IE by causing inflammation and cytotoxicity directly at the site by: 1) destabilizing plasma membranes, and 2) generating by-products from sphingosine digestion. Previous work has shown β-toxin is capable of upregulating pro-inflammatory cytokines exaggerating the host inflammatory response which would lead to a subsequent influx of host immune cells and host factors to repair and control the damage.19 The resulting inflammation would serve as a better platform to which S. aureus could bind, leading to macrocluster formation and increasing vegetation size. While β-toxin is not a pore forming toxin, it has been shown to affect host cell plasma membranes including lysing blood erythrocytes and epithelial cells, which could lead to inflammation.20 As a SMase, β-toxin digests sphingomyelin into phosphorylcholine and ceramide, a chemotactic signal. Studies have shown ceramide inhibits angiogenesis, induces apoptosis of various cardiac cell types, and might favor thrombosis.21, 22 Additionally, activation of a neutral SMase in aortic endothelial cells is associated with heart failure and endothelial dysfunction.22 Given the data showing ceramide has detrimental effects on the heart, it is possible the decrease in vegetation size observed with the SMase mutant may be linked to the ceramide this activity produces as a result of sphingomyelin digestion. Other by-products of sphingomyelin digestion are also involved in signaling and may also contribute to vegetation formation.
Consistent with its ability to bind to DNA and possibly other host factors aiding in biofilm formation, β-toxin also increases vegetation size via its DNA biofilm ligase activity. However, SMase mutant-infected rabbits show decreased vegetation size despite having the DNA biofilm ligase activity still intact. This may be because the initial effects the SMase activity provides directly at the site are no longer present and might be necessary to initiate vegetation growth. Thus, without SMase activity there may not be sufficient vegetation formation to begin with for the DNA biofilm ligase activity to build upon. Following initial vegetation formation, the DNA biofilm ligase activity may quickly build up the vegetation before it breaks off to stabilize colonization by attaching to the host factors the SMase activity induced inflammation attracts.
It is important to note that while the DNA biofilm ligase activity increases vegetation size, it does not affect bacterial replication within the vegetation as can be seen by the similar CFUs recovered from mutants and wild type. This is consistent with the findings of Salgado-Pabón et al. and O’Callaghan et al.1, 23, who despite finding increased pathogenesis in tissues with β-toxin, did not see an increase in CFUs recovered. The DNA biofilm ligase activity might serve to build up the vegetations so S. aureus is protected from stresses such as host immune defenses and to decrease its susceptibility to antibiotics. Biofilm dispersal and sensitivity to antimicrobials have been shown to increase with degradation of extracellular DNA.24 It is possible S. aureus is using the DNA biofilm ligase activity to bind extracellular DNA to shield it from enzymatic degradation. β-toxin may also be binding to extracellular DNA to increase its proximity to other DNA binding proteins to further prevent DNA degradation.
Additional studies on endothelial cells and histopathology need to be done to determine if inflammation or cytotoxicity are present and responsible for the pathogenesis observed. Characterization of how β-toxin SMase activity induces inflammation and what host factors the DNA biofilm ligase active site binds to may provide targets for therapeutic treatments.
Supplementary Material
Acknowledgments
Funding Sources
This research was supported by a start-up grant by the University of Iowa to PMS.
Dr. Jeffrey Kavanaugh is gratefully acknowledged for assistance in making 3-D models of β-toxin and mutants
ABBREVIATIONS
- CFUs
colony-forming units
- IE
infective endocarditis
- IPTG
isopropyl-β-D-thiogalactopyranoside
- LD50
lethal dose 50% endopoint
- MSCRAAMs
microbial surface components recognizing adhesive matrix molecules
- PBS
phosphate-buffered saline
- SAgs
superantigens
- SMase
sphingomyelinase
- TSB
tryptic soy broth
- TB
terrific broth
Footnotes
Author Contributions
All authors contributed to data collection. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Figure Supplement 1 shows all strains used in the biofilm formation assay grew to similar absorbance 600 nm wavelength in wells for biofilm assays and representative wells of biofilm growth for each strain, stained with crystal violet. Additionally, there was no apparent defect or difference in growth between the strains as seen by a growth curve. Figure Supplement 2 shows COL strains carrying β-toxin or a β-toxin mutant on a plasmid expressed high levels of the protein, quantified by measuring zones of hemolysis compared to a standard curve made by recombinant β-toxin. It also shows representative images of heart vegetations recovered from rabbits infected at the lower inoculum. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Salgado-Pabon W, Herrera A, Vu BG, Stach CS, Merriman JA, Spaulding AR, Schlievert PM. Staphylococcus aureus beta-toxin Production is Common in Strains with the beta-toxin Gene Inactivated by Bacteriophage. The Journal of infectious diseases. 2014 doi: 10.1093/infdis/jiu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huseby MJ, Kruse AC, Digre J, Kohler PL, Vocke JA, Mann EE, Bayles KW, Bohach GA, Schlievert PM, Ohlendorf DH, Earhart CA. Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14407–14412. doi: 10.1073/pnas.0911032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katayama Y, Baba T, Sekine M, Fukuda M, Hiramatsu K. Beta-hemolysin promotes skin colonization by Staphylococcus aureus. Journal of bacteriology. 2013;195:1194–1203. doi: 10.1128/JB.01786-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spaulding AR, Satterwhite EA, Lin YC, Chuang-Smith ON, Frank KL, Merriman JA, Schaefers MM, Yarwood JM, Peterson ML, Schlievert PM. Comparison of Staphylococcus aureus strains for ability to cause infective endocarditis and lethal sepsis in rabbits. Frontiers in cellular and infection microbiology. 2012;2:18. doi: 10.3389/fcimb.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferro JM, Fonseca AC. Chapter 7 - Infective endocarditis. In: José B, José MF, editors. Handbook of Clinical Neurology. Elsevier; 2014. pp. 75–91. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A, Lomas JM, Galvez-Acebal J, Hidalgo-Tenorio C, Ruiz-Morales J, Martinez-Marcos FJ, Reguera JM, de la Torre-Lima J, de Alarcon Gonzalez A. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013;127:2272–2284. doi: 10.1161/CIRCULATIONAHA.112.000813. [DOI] [PubMed] [Google Scholar]
- 7.Hoen B, Duval X. Infective Endocarditis. New England Journal of Medicine. 2013;368:1425–1433. doi: 10.1056/NEJMcp1206782. [DOI] [PubMed] [Google Scholar]
- 8.Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovascular Pathology. 2006;15:256–263. doi: 10.1016/j.carpath.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013;26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Independent investigation by Immuven Inc.
- 11.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA: the journal of the American Medical Association. 2005;293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 13.Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, Barriere S, Woods CW, Chu VH, Marin M, Bukovski S, Garcia P, Corey GR, Korman T, Doco-Lecompte T, Murdoch DR, Reller LB, Fowler VG, Jr International Collaboration on Endocarditis-Microbiology, I. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. The Journal of infectious diseases. 2011;204:704–713. doi: 10.1093/infdis/jir389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, Bohach GA, Schlievert PM, Ohlendorf DH, Earhart CA. Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol. 2007;189:8719–8726. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Frontiers in cellular and infection microbiology. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. Nuclease Modulates Biofilm Formation in Community-Associated Methicillin-Resistant Staphylococcus aureus. PloS one. 2011;6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, Wang F, Brosnahan AJ, Mleziva JA, Peterson ML, Jones BE. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry. 2007;46:14349–14358. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annual review of microbiology. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 19.Walev I, Weller U, Strauch S, Foster T, Bhakdi S. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infection and immunity. 1996;64:2974–2979. doi: 10.1128/iai.64.8.2974-2979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cifrian E, Guidry AJ, Bramley AJ, Norcross NL, Bastida-Corcuera FD, Marquardt WW. Effect of staphylococcal beta toxin on the cytotoxicity, proliferation and adherence of Staphylococcus aureus to bovine mammary epithelial cells. Veterinary microbiology. 1996;48:187–198. doi: 10.1016/0378-1135(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Förstermann U. Dual Effect of Ceramide on Human Endothelial Cells: Induction of Oxidative Stress and Transcriptional Upregulation of Endothelial Nitric Oxide Synthase. Circulation. 2002;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 22.Levade T, Auge N, Veldman RJ, Cuvillier O, Negre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circulation research. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, Engel LS, Hill JM. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infection and immunity. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okshevsky M, Regina VR, Meyer RL. Extracellular DNAas a target for biofilm control. Current opinion in biotechnology33. 2015:73–80. doi: 10.1016/j.copbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.