Fig.6. ELISAs with patient plasma.
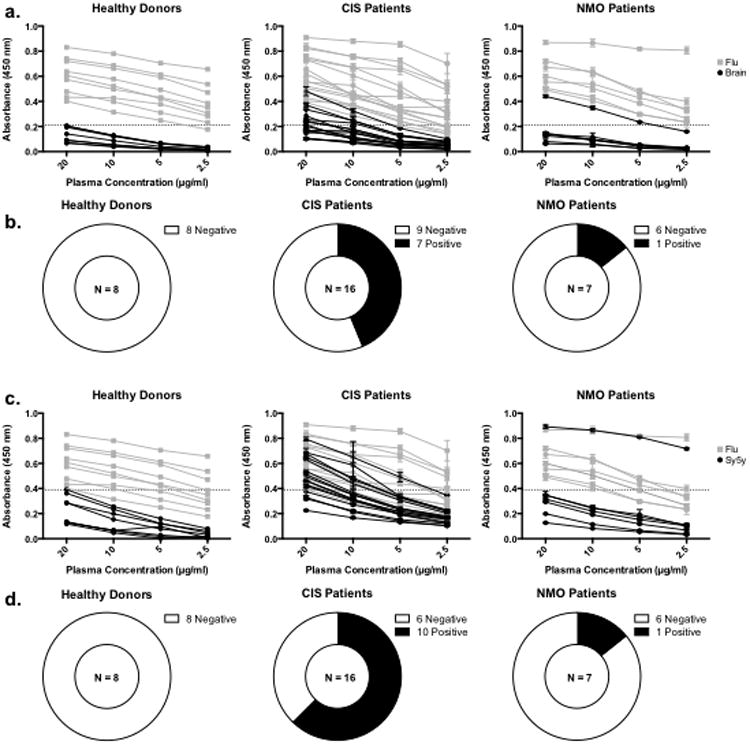
ELISA results with plasma taken from 8 healthy donors responding to influenza vaccination, 16 treatment naïve CIS-PTM patients, and 7 NMO patients. (a.) Absorbance data from plasma ELISAs on brain lysate and influenza antigen grouped by patient classification. Each black line represents ELISA data from an individual plasma antibody sample tested on brain lysate while each gray line represents ELISA data from an individual plasma antibody sample tested on influenza antigen. The dashed line on each graph represents the cutoff for positive binding (the average absorbance from healthy donor plasma on brain lysate at 20 μg/mL plus two standard deviations). (b.) Summary of brain lysate ELISAs with plasma antibodies. Plasma antibody samples from 1 NMO and 7 CIS-PTM patients were two standard deviations above the mean of healthy plasma antibody samples at 20 μg/mL. (c.) Absorbance data from plasma ELISAs on SH-Sy5y lysate and influenza antigen grouped by patient classification. Data are presented as in a, except that SH-Sy5y data is displayed in black. (d.) Summary of SH-Sy5y lysate ELISAs with plasma antibodies. Plasma antibody samples from 1 NMO and 10 CIS-PTM patients were two standard deviations above the mean of healthy plasma samples at 20 μg/mL.
