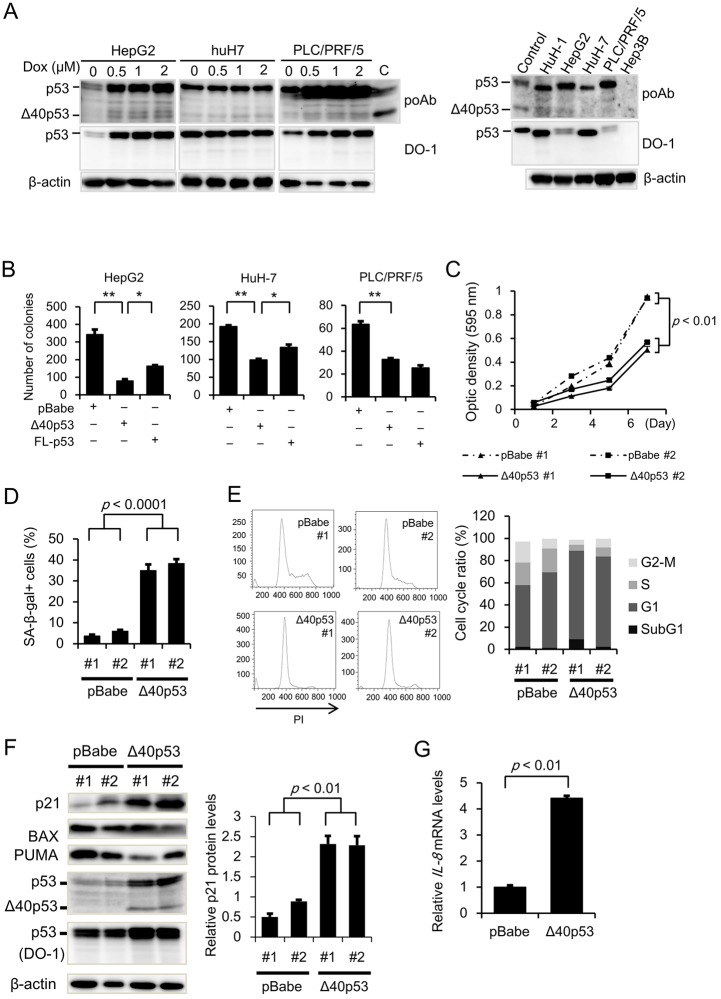
Fig. 3.
Effect of exogenous Δ40p53 expression on cell growth and senescence. (A) Protein expression levels of Δ40p53 in HCC cells. Left panel, inducible protein expression of Δ40p53. HepG2, PLC/PRF/5, and HuH-7 cells were incubated in medium containing the indicated concentration (0, 0.5, 1, 2 μM) of doxorubicin (Dox) for 12 h; right panel, endogenous protein expression of Δ40p53 in HCC cell lines including HuH-1 (TP53WT), HepG2 (TP53WT), PLC/PRF/5 (TP53R249S), HuH-7 (TP53Y220C), and Hep3B (TP53−/−) cells. The cells were lysed with lysis buffer; 5 or 1 μg of protein lysate was then subjected to western blot analysis to detect p53 or β-actin protein, respectively. An anti-p53 polyclonal antibody (poAb) and an anti-p53 monoclonal antibody (DO-1) were used to detect both FL-p53 and Δ40p53. C, control (HepG2 TP53+/Δ40 cells). (B) Effect of Δ40p53 on clonogenicity. pBabe, Δ40p53/pBabe, and FL-p53/pBabe retroviruses were generated using 293T cells. After the viral supernatants were prepared, HepG2 cells, PLC/PRF/5, and HuH-7 cells were infected at the same MOI with pBabe, Δ40p53/pBabe, or FL-p53/pBabe. After infection for 48 h, the cells were treated with puromycin (2 μg/ml) for 14 days followed by staining with Crystal Violet and imaging. Bar graphs represent the number of stained colonies (n=6). *P<0.05; **P<0.005. (C) The growth rate of the pBabe and Δ40p53/pBabe HepG2 cell clones were determined by using an MTT assay. Each single clone was picked from a dish after 14 days of puromycin treatment, expanded, and then utilized for the following assay. The optical density (595 nm) at each time point (day 0, 2, 4, and 6) is presented as the mean±s.e.m. (n=4). (D) The cellular senescence of the pBabe and Δ40p53/pBabe clones was examined as described in the legend of Fig. 1E. Bar graphs represent the percentage of SA-β-gal-positive cells (mean±s.e.m.; n=3). (E) Cell cycle analysis of pBabe and Δ40p53/pBabe clones. Each cell clone (105 cells/well) was incubated in serum-free medium for 48 h to synchronize the cell cycle. After 48 h of serum starvation, the cells were further cultured in medium containing 10% FBS for 24 h. The cells were detached, fixed and stained with propidium iodide (PI, 100 μg/ml) after RNase (1 mg/ml) treatment. The cell cycle populations were measured by FACS. A representative FACS histogram and bar graphs of the cell cycle ratios are shown. (F) Protein levels of p21, BAX, PUMA, Δ40p53 and FL-p53 in the pBabe and Δ40p53/pBabe clones were determined by western blot analysis as described in the legend of Fig. 1F. (G) qRT-PCR analysis of relative IL-8 gene expression level in pBabe and Δ40p53 clones. The data are expressed relative to the mRNA levels found in the corresponding sample of pBabe clones, which was arbitrarily defined as 1.