Abstract
Overview
This paper describes the study protocol used to evaluate the Resilient, Empowered, Active Living with Diabetes (REAL Diabetes) intervention and reports on baseline characteristics of recruited participants. REAL Diabetes is an activity-based intervention designed to address the needs of young adults diagnosed with type 1 (T1D) or type 2 diabetes (T2D) from low socioeconomic status or racial/ethnic minority backgrounds. The REAL intervention incorporates tailored delivery of seven content modules addressing various dimensions of health and well-being as they relate to diabetes, delivered by a licensed occupational therapist.
Methods
In this pilot randomized controlled trial, participants are assigned to the REAL Diabetes intervention or an attention control condition. The study’s primary recruitment strategies included in-person recruitment at diabetes clinics, mass mailings to clinic patients, and social media advertising. Data collection includes baseline and 6-month assessments of primary outcomes, secondary outcomes, and hypothesized mediators of intervention effects, as well as ongoing process evaluation assessment to ensure study protocol adherence and intervention fidelity.
Results
At baseline, participants (n=81) were 51% female, 78% Latino, and on average 22.6 years old with an average HbA1c of 10.8%. A majority of participants (61.7%) demonstrate clinically significant diabetes distress and 27.2% report symptoms consistent with major depressive disorder. Compared to participants with T1D, participants with T2D had lower diabetes-related self-efficacy and problem-solving skills. Compared to participants recruited at clinics, participants recruited through other strategies had greater diabetes knowledge but weaker medication adherence.
Discussion
Participants in the REAL study demonstrate clinically significant medical and psychosocial needs.
Keywords: Randomized controlled trial, health behavior, diabetes mellitus, occupational therapy, young adult
1. Introduction
Type 1 diabetes (T1D) is the second most common serious childhood disease in the United States, and type 2 diabetes (T2D) has been identified as an “emerging epidemic” (1) in youth, particularly youth from racial/ethnic minority backgrounds (2). Among young people with diabetes, as with other chronic conditions, the transition to adulthood is a particularly challenging time for disease management. Treatment adherence is low and few young adults attain optimal levels of blood glucose control, increasing the risk of developing both acute and long-term complications of diabetes (3, 4). Indeed, recent epidemiological data in T1D indicates that only 13% of young adults age 18–25 years attain optimal levels of glycemic control (as indicated by an A1C level of <7%, the recommendation for most healthy adults with diabetes), and young adults self-monitor their blood glucose levels less frequently than individuals in any other age group (4). Furthermore, young adults with diabetes have higher rates of mental illness, substance abuse, and mortality than both their peers without diabetes and older populations with diabetes (3, 5–10).
Despite these serious concerns, only a handful of empirical studies have evaluated interventions designed to improve disease management and overall well-being amongst young adults (11–16). Among these studies, only one specifically addressed the diabetes care challenges of low-socioeconomic status (SES) or minority populations (11), and only two included young adults with T2D (13, 15). Further, most interventions addressing young adults’ diabetes care focus primarily on facilitating the transition from pediatric to adult healthcare systems. While ensuring a smooth transition between healthcare systems is indeed critical to ensure that young adults receive needed services, this approach does not address the wide array of challenges that young adults encounter in performing diabetes self-management outside of a clinical context.
In light of the identified gaps in research on diabetes care among young adults, particularly those who are from low-SES or racial/ethnic minority backgrounds, our research group has developed, and is currently pilot testing, an activity-based diabetes management intervention called Resilient, Empowered, Active Living with Diabetes (REAL Diabetes). The intervention framework is based on principles of Lifestyle Redesign, a preventive occupational therapy intervention originally developed to address the needs of community dwelling, ethnically diverse older adults (17, 18), which has since been adapted to address health-promoting lifestyle change in a variety of populations (19, 20). The Lifestyle Redesign intervention framework has been shown in two large-scale randomized controlled trials (RCTs) to cost-effectively improve physical and mental well-being, and facilitate maintenance of therapeutic gains over the long term (17, 18, 21, 22). To adapt this framework to address the needs of the targeted population, our research team conducted a qualitative needs assessment amongst young adults with both T1D and T2D (23, 24), and developed and validated an intervention manual among a small sample of young adults with diabetes (25). After completing intervention development and validation, our research team initiated a pilot RCT, described herein, to evaluate the efficacy of the intervention and further optimize its implementation in preparation for a large-scale RCT.
2. Research design and methods
2.1. Study design
The primary aim of the REAL Diabetes Study is to evaluate the efficacy of an occupation-based diabetes management intervention on improving the self-care behaviors and glycemic control of young adults with T1D or T2D. Secondary aims include conducting exploratory analyses to examine potential mechanisms that may explain the intervention’s effects, and evaluating the intervention’s impact on secondary outcomes including diabetes-related distress, diabetes-related quality of life, depression, and life satisfaction. Finally, we will conduct a rigorous process evaluation utilizing mixed methods to evaluate and refine both the REAL intervention and overall study procedures.
2.2. Recruitment
Participants were recruited through a variety of clinical and community settings. These included in-person recruitment at diabetes clinics; fliers and brochures placed in diabetes and primary care clinics; mass mailings to patients at diabetes clinics; health fairs; community college newspaper advertising; social media advertising; and word-of-mouth referrals from friends or family members.
2.3. Eligibility criteria
Eligible participants were adults age 18–30 years old, with a diagnosis of either T1D or T2D for at least 12 months, and a hemoglobin A1C of ≥8.0% at the time of study enrollment. Participants were fluent in English or Spanish, were reachable by phone or text message, lived within Los Angeles County with no plans to relocate, and expressed willingness to participate in all study-related activities. While study funds were allocated to provide cell phones with text messaging capability to participants who did not have cell phones or who could not afford the additional cost of calling or text messaging the study team using their personal cell phone, all participants declined this offer. All participants were low in socioeconomic status; initially this was operationalized to mean that the participant had a household income of 138% of Federal Poverty Level or lower. This definition was later expanded to include the following: (1) the participant qualified for income-restricted government entitlement programs (e.g., MediCal/Medicaid, Supplemental Nutrition Assistance Program); (2) the participant had a household income of 250% of Federal Poverty Level or lower; or (3) neither of the participant’s parents had attained a four-year college degree. Participants were excluded if they had cognitive disabilities or severe impairments that limited life expectancy; were pregnant or planned a pregnancy within the next 6 months; had participated in a diabetes management intervention beyond diabetes education offered in usual clinic visits totaling ≥6 hours within the past year; or if they had participated in qualitative research used to inform the development of the REAL Diabetes intervention (23, 24).
2.4. Enrollment & randomization
Initial contact with participants took place either in person at participating clinics or via email and phone. To initiate enrollment at clinics, permission was obtained through the Institutional Review Board (IRB) to conduct preliminary chart reviews to prescreen for provisional eligibility criteria only. Patients who were provisionally eligible per medical chart review were then approached in clinic and invited to participate. After being informed about the study’s purpose and procedures, prospective participants completed the full eligibility screening and, if time allowed, completed enrollment procedures including informed consent, a HIPAA authorization form, and providing contact information. Subsequently, participants were contacted to schedule an in-person session in their home or another location of their choosing to administer baseline assessments.
In order to extend recruitment to young adults not seen at participating clinics, study advertisements and brochures were displayed in paper format in public community spaces, such as colleges and recreation centers, as well as electronically through social media. Potential participants who contacted the study to express interest in the study were screened for eligibility over the phone by study staff. Those who were provisionally eligible based on their self-report responses were scheduled for an in-person eligibility screening (including point-of-care A1C testing) and completion of enrollment procedures. Participants who were found to be eligible at the in-person screening, and who completed enrollment procedures, then completed baseline assessments in the same session.
Participants were randomized immediately following baseline testing and entry of baseline data into the computerized Research Electronic Data Capture (REDCap) database management system (26). Participants were notified of their assigned intervention group immediately after randomization, and contacted by the corresponding interventionist within two business days to schedule their initial intervention visit. Randomization was stratified by type of diabetes (type 1 or type 2) using random block sizes. Within each stratum and block, equal numbers of participants were assigned to each treatment group in random order. The master randomization list was generated by a SAS program which, along with the randomization list, was securely maintained on the statistician’s network drive. The randomization list was uploaded to REDCap and automatically assigned participants when the eligibility checklist form was complete. Randomized assignment was available only to specified study personnel through the REDCap system. Initially, all participants were randomized through the REDCap system. However, an inadvertent programming error resulted in the type 2 stratum being deleted from the database. Although all data were fully restored, the automated randomization module could not be reset to the appropriate point, and subsequent type 2 participants were randomized manually by opening sealed, opaque envelopes prepared by the study’s biostatistician.
2.5. Intervention
2.5.1. Attention Control Intervention
The attention control intervention is delivered by a graduate public health student and a promotora de salud (community health worker), both of whom are blinded to the study’s outcome measures. Participants in the attention control condition receive a packet of standardized educational materials from the National Diabetes Education Program, National Institutes of Health (NIH), and USDA MyPlate.gov, as well as a listing of local health centers in their area; the focus of educational materials is outlined in Table 1. This packet is delivered in-person to the participant’s home or a community location of their choosing. The home visit lasts approximately 15 minutes and includes an introduction to the educational materials and review of the intervention procedures. Subsequently, at approximately 2-week intervals, participants receive a phone call from the intervener in which they are asked if they have reviewed specific parts of the packet and if they have any questions related to the educational materials. The interveners reinforce and clarify information in the educational materials, and refer any questions outside the scope of the materials to the participant’s healthcare provider.
Table 1.
Intervention Content
| Module | REAL Intervention Content | Attention Control – Information Packet |
|---|---|---|
| 1. Assessment and Goal-Setting |
|
|
| 2. Living with Diabetes |
|
|
| 3. Access and Advocacy |
|
|
| 4. Activity and Health |
|
|
| 5. Social Support |
|
|
| 6. Emotions and Wellbeing |
|
|
| 7. Long Term Health |
|
|
2.5.2. REAL Diabetes Intervention
The REAL Diabetes Intervention is delivered by two licensed occupational therapists, both of whom are blinded to the study’s outcome measures. The intervention draws on the expertise of occupational therapy in evaluating the fit between demands of everyday activities (e.g., health management activities involved in diabetes care) and the skills and abilities of patients, and devising strategies to enable participation in these activities. The role of occupational therapy in chronic disease management has been described as facilitating patients’ consistent, habitual, and correct performance of health management tasks through the integration of these tasks into daily routines (27). The emphasis on developing consistent diabetes self-care habits and routines forms the overarching focus of the intervention. Prior to initiating the intervention, each therapist received 20 hours of training in the intervention manual, 20 continuing education hours in diabetes education, and 12 hours of motivational interviewing training. The REAL intervention is delivered in approximately 12 biweekly sessions averaging one hour each, although therapists have discretion to customize the timing and duration of sessions, with the aim of delivering an intervention dose of 10–16 treatment hours over a 6-month period. Intervention sessions take place in participants’ homes or community locations of their choosing. Therapists also utilize text messaging to deliver information, reminders, and engage in brief clinical problem-solving conversations between in-person sessions. For example, as habits are heavily emphasized in the intervention, and habits rely on contextual cues, therapists assist participants to adjust cues if the initial cue is not successful (e.g., leaving bedtime medication on the nightstand as a visual cue, versus setting an alarm at bedtime as a reminder to take medication).
The REAL intervention manual includes an overview of the intervention protocol and theoretical framework, and seven content modules as outlined in Table 1. Each module includes suggested goals, suggested activities to support those goals, and client handouts and resources. The intervention is individually tailored, insofar as only the first and last module are utilized with all participants; content from the remaining modules is utilized as needed to support participants’ individual treatment goals. The intervention is also developmentally tailored in both its content and modes of delivery. Intervention modules contain content relevant to lifestyle-related concerns typical of young adults such as managing diabetes in the context of changing work and school schedules, how alcohol and substance use affect blood sugar, and how to access and communicate effectively with adult healthcare providers. Communication between in-person sessions is primarily via text messaging, as has been the preference expressed by the majority of young adult participants in our previous studies.
The intervention manual exists as both a hard copy and digitally on a web-enabled tablet to facilitate real-time access to internet resources during treatment sessions. It emphasizes activity-based intervention strategies such as role-playing, environmental modification, or direct engagement in activity (such as preparing a meal to improve carbohydrate counting skills, or exploring diabetes-related blogs to develop a support network of people with diabetes).
Intervention fidelity is maintained through three strategies. First, therapists document their adherence to the intervention protocol in treatment notes for each intervention session. Second, approximately 10% of sessions are observed by another therapist trained in the intervention, who completes a fidelity checklist and provides feedback to the treating therapist. Finally, weekly meetings are held with the full intervention team to facilitate problem-solving and prevent intervention drift.
2.6. Data collection and data management
Data are collected via in-person sessions at participants’ homes or community settings of their choosing, as well as via medical chart review. In-person testing takes place at baseline and 6 months, with testing sessions lasting approximately 90 minutes on average. Participants receive $25 at the baseline testing session, and $50 at the 6-month testing session. Data collectors are blinded to participants’ group assignment. Medical chart reviews, retrospective over a 12-month period, take place at baseline and 12 months to obtain clinical and healthcare utilization data, including A1C values and number of routine diabetes care visits (defined as any visit to a primary care provider or endocrinologist where an A1C value was recorded, or where diabetes appears in the problem list). For participants recruited from clinics, medical records at these clinics were reviewed. Additionally, all participants were asked for the names and contact information of any primary care providers and endocrinologists they had seen during the past year, and medical records were requested from these providers. A full list of study measures collected by time point is provided in Table 2. In addition, intervention delivery and process evaluation measures are collected utilizing a separate database visible to therapists only.
Table 2.
List and timeline of study measures
| Construct | Assessment | Description | Time Administered | ||
|---|---|---|---|---|---|
| Screening/Enrollment | Baseline | Follow-up (6 months unless indicated) | |||
| N/A | Screening log | Demographic characteristics of those approached (gender; age; ethnicity); enrollment status | X | ||
| N/A | Eligibility checklist | Documentation of eligibility criteria | X | ||
| N/A | Contact information | Address, phone number, email; emergency contact information; communication preferences | X | ||
| N/A | Medical chart review | Date of birth; vital signs; diabetes treatment regimen; comorbidities; family history; social history | X | X (12 months) | |
| N/A | Demographic questionnaire | Gender; ethnicity; education; country of origin; household composition; current source of medical care; family history of diabetes | X | X (partial) | |
| Stressful life events | Stressful life event checklist | 24 items compiled from childhood trauma and life event checklists (28–32); evaluates exposure to stressful life events | X | ||
| Drug and alcohol use | CAGE Questions Adapted to Include Drugs (CAGE-AID) (33) | 4 items; assesses possible drug or alcohol dependence | X | ||
| Glycemic control | A1C (Axis-Shield Afinion point-of-care assay) | Meets NGSP certification criteria; sensitive to A1C scores up to 15.0% (scores above this were recorded as 15.1%); correlation with laboratory A1C measure = 0.991 (34, 35) | X | X | |
| Diabetes self-care | Summary of Diabetes Self-Care Activities (SDSCA) (36) | 14 items; evaluates diet, physical activity, blood glucose monitoring, medications, foot care, and smoking | X | X | |
| Diabetes-related quality of life | Audit of Diabetes-Dependent Quality of Life (ADD-QoL) (37) | 19 items; assesses social, physical, and emotional functioning | X | X | |
| Diabetes-related self-efficacy | Diabetes Empowerment Scale-Short Form (DES-SF) (38) | 8 items; measures psychosocial self-efficacy | X | X | |
| Diabetes knowledge | Diabetes Knowledge Questionnaire (DKQ-24) (39) | 24 items; measures general diabetes knowledge | X | X | |
| Problem-solving | Diabetes Problem-Solving Inventory (DPSI) (40) | Structured interview; probes for problem solving strategies for 9 scenarios regarding blood glucose monitoring, stress management and taking medications | X | X | |
| Diabetes distress | Problem Areas in Diabetes-Short Form (PAID-5) (41) | 5 items; measures diabetes-related distress and emotional problems | X | X | |
| Depressive symptoms | Patient Health Questionnaire (PHQ-8) (42) | 8 items; assesses depressive symptom severity | X | X | |
| Activity participation | Participation Objective Participation Subjective (POPS) (43) | 26 items; measures participation frequency, importance, and desired change | X | X | |
| Habit strength | Self-Report Behavioral Automaticity Index (SRBAI) – blood glucose monitoring and taking medication (44) | 4 questions for each behavior; assesses automaticity for blood glucose testing and taking insulin/medications | X | X | |
| Life satisfaction | Satisfaction with Life Scale (SWLS) (45) | 5 items; measures global life satisfaction/subjective well-being | X | X | |
| N/A | Exit interview | 15-minute interview to assess satisfaction with intervention and study participation | X | ||
In collaboration with the Southern California Clinical and Translational Science Institute (SC CTSI), a REDCap database was developed and utilized for data management in this study (26). REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. This database system also facilitates provision of data to the IRB and NIH. All assessment data were double entered and reconciled by research assistants blinded to participants’ group assignment.
2.6.1. Demographic measures
Participants’ health information, including date of birth, BMI, blood pressure, lipids, date of diabetes diagnosis and treatment regimen, and occurrences of diabetes complications and comorbid conditions are evaluated at baseline via medical chart review. Participant demographics, including gender, race, ethnicity, education, generational status, household composition, drug and alcohol use, and past and on-going stressful life events, are attained at baseline via self-report questionnaires. Evaluating individual-level socioeconomic status is challenging among young adults because socioeconomic status is typically indexed via measures of income, educational attainment and occupation. However, during the age range of 18–30, these variables are in a state of flux, as individuals are often enrolled in secondary and post-secondary education and transitioning among various employment situations. Therefore, parental education and occupation data were collected as measures of household socioeconomic status. Following the procedures outlined in Hollingshead (47), parental occupation was coded on a 1 to 9 scale and education was coded on a 1 to 7 scale. When both parents’ occupation and education were provided, the values were averaged. Average occupation scores were weighted by 5 and education scores weighted by 3, and the product of these values was calculated, producing an overall Hollingshead score ranging from 8 to 66. In addition to household-level socioeconomic data, participants’ home addresses were used to look up Census tract data including median household income and the percentage of residents living below the poverty level.
2.6.2. Outcome measures
The study’s primary clinical outcome, hemoglobin A1C, is obtained using a point-of-care device (Axis-Shield Afinion) which collects capillary blood using a standard finger-prick procedure. In cases of device malfunction, a laboratory A1C value from within two weeks of the participant’s testing date is substituted. The study’s primary behavioral outcome, adherence to diabetes self-care behaviors, is evaluated via the Summary of Diabetes Self-Care Activities scale (SDSCA) (36). Secondary outcomes, including diabetes-related distress, diabetes-related quality of life, depression, and life satisfaction, are evaluated using self-report measures as outlined in Table 2. Finally, routine diabetes care utilization is evaluated via medical chart review at 12 months, with routine visits defined as those in which hemoglobin A1C is assessed.
2.6.3. Additional measures
Hypothesized mediators of the intervention, which include habit strength for taking medication and self-monitoring blood glucose, diabetes knowledge, diabetes self-efficacy, diabetes-related problem solving, and satisfaction with daily activities, are evaluated using the self-report measures outlined in Table 2. Process evaluation measures are collected throughout the study to evaluate and refine the intervention and study procedures. These include data on intervention adherence, protocol deviations, adverse events, and intervention fidelity. Therapists maintain a separate documentation log, which includes the duration of each session, clinical issues addressed, and activities used with participants. In addition, text messages are utilized to schedule baseline and 6-month testing sessions, as well as a communication tool during the intervention. Intervention-related text messages are tracked using an online system in order to evaluate the dose and context of each text. Finally, participants complete a brief exit interview at their 6-month testing session, in which they provide subjective feedback on their experience with the REAL intervention or attention control intervention. This interview is conducted after all outcome variables have been assessed, with the interview guide placed in a sealed envelope to maintain testers’ blinding to condition assignment until after follow-up data are collected.
2.7. Analytic plan
2.7.1. Sample size justification
The study is powered on an intention-to-treat analysis of mean change in hemoglobin A1C at 6 months as compared to baseline. Sample size calculation was based on a two-tailed alpha level of 0.05, 90% power, and 15% attrition. We estimated a 0.8% between-group difference in A1C, assuming a pooled standard deviation of 1%. Based on these parameters, a calculation using G*Power software yielded an estimate of 80 participants (40 per group).
2.7.2. Primary analyses
The primary analyses will evaluate intervention efficacy for mean change in A1C and diabetes self-care (testing blood glucose and taking medication) on an intention-to-treat basis using a Student’s t-test assessing between-group differences. Signed change scores will be calculated for each variable by subtracting each participant’s baseline values from his or her post-intervention values. To assess the robustness of these analyses, we will compare results including and excluding outliers, and with and without imputed data (multiple imputation of baseline or follow-up values as necessary; SAS proc mi), to assess whether these data are driving trends observed in the results. Baseline data for A1C, glucose monitoring and medication adherence indicate no outliers and only one missing medication adherence value. Additionally, we will conduct exploratory analyses using ANCOVA methods to examine whether any demographic variables were effect modifiers which should be considered as potential stratifying variables in future studies.
2.7.3. Secondary analyses
We will evaluate the intervention’s impact on secondary outcomes and potential mediators of intervention efficacy. Analysis of secondary outcomes will investigate between-group differences in change on measures of depression, life satisfaction, diabetes-related quality of life, and diabetes-related stress. Analysis of potential mediators will assess between-group differences in changes on measures of habit strength, problem solving, activity participation, diabetes knowledge, and diabetes self-efficacy, and the association of changes in each of these measures with the primary outcomes. Although the pilot study lacks power to test a full meditation model, our aim is to learn which potential mediators change as a result of the intervention, and which potential mediators are associated with changes in A1C and diabetes self-care. These data will inform modification of the intervention and facilitate power calculations to evaluate mediating pathways using structural equation modeling in a large-scale trial of the intervention.
2.7.4. Analyses for current paper
In this paper, we report on participants’ baseline demographic, clinical, and psychosocial characteristics, both for the sample overall and comparing three key strata: participants with T1D versus T2D, men versus women, and participants recruited in-person at clinical sites versus those recruited through mass mailings or social media advertising. All data were analyzed using SAS for Windows, version 9.4 (SAS Institute, Cary, N. C.). All p-values are two-sided. Between-group differences were evaluated using Fisher’s exact tests and Wilcoxon rank-sum tests as appropriate.
3. Results
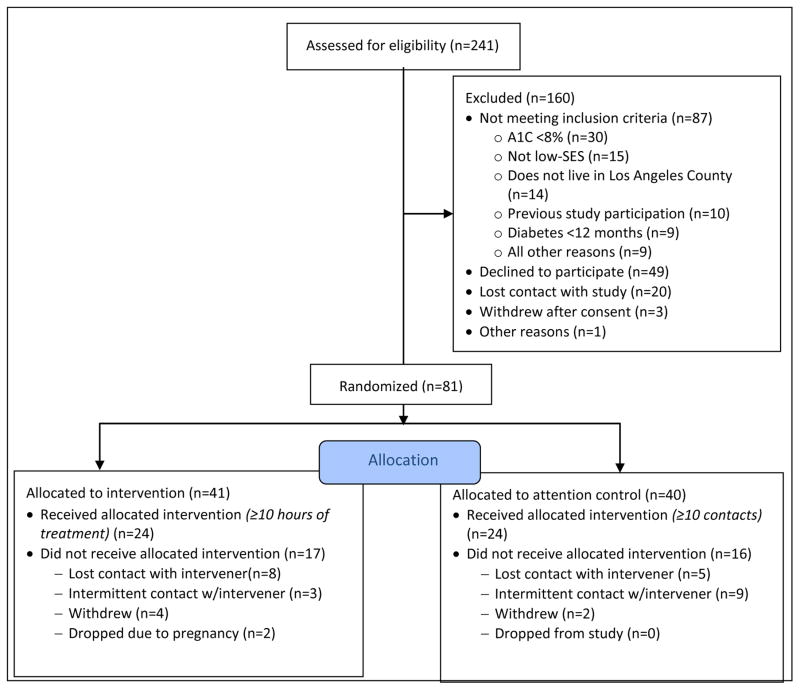
3.1. Recruitment flow
Figure 1 shows participants’ recruitment, randomization and treatment allocation as of 4/15/2016. Recruitment began in October 2014 and was completed in December 2015. Recruitment was carried out in two waves, the first beginning in October 2014 and the second beginning in April 2015. The first wave of recruitment included primarily in-person contact with potential participants at two hospitals: a major urban children’s hospital and a large public hospital in Los Angeles County. Within these hospitals, study personnel attended pediatric and young adult outpatient diabetes clinics and approached patients to provide information about the study and conduct eligibility screenings.
Figure 1.
Enrollment Diagram
The second wave of recruitment, initiated in response to ongoing recruitment challenges at the children’s hospital and emerging challenges at the public hospital, was conducted primarily through mass mailings and social media advertising. The mass mailing was directed to patients at the children’s hospital: in-person recruitment was discontinued and instead, all patients at the hospital age 18 and older with a diagnosis of diabetes were mailed a letter and study brochure. The mailing was sent in three waves, in April, June, and July 2015. In October 2015, a follow-up mailing was sent to a randomly-selected 60% of the original mailing list. Social media advertisements on Facebook and Twitter were targeted by age, geographic region, and user-identified interests such as diabetes information and support websites. Prospective participants were referred to a website where they could view and download informational materials about the study, and submit their contact information for follow-up by a member of the study team.
3.2. Retention
As of August 23, 2016, 71 (of 81 total) randomized participants completed follow-up testing. Of the 10 participants who did not complete follow-up testing, nine participants (11.1%) were lost to follow-up, and one participant (1.2%) withdrew from the study, citing a lack of time to complete the study requirements.
3.3. Participant characteristics
Table 3 presents baseline characteristics for the sample as a whole, as well as for participants with T1D versus T2D and those recruited from clinics versus via social media and mailings (SM/M). In comparing baseline characteristics between treatment and control groups, the only significant difference was in family history of diabetes: participants in the control group were more likely to have an extended family member with diabetes than those in the treatment group (92% vs. 68%, p=0.01). As treatment groups were randomly assigned, this is a chance occurrence, the likes of which are not unexpected with multiple comparisons. Family history of diabetes was not found to be associated with baseline values of A1C in the total sample or either of the randomized groups (p>0.43 for all). Additionally, an analysis examining differences in baseline characteristics according to gender showed that women, as compared to men, had higher depressive symptoms (PHQ-8 scores 6.9 ± 5.9 vs. 4.7 ± 3.6, p=0.05).
Table 3.
Demographic, clinical and psychosocial characteristics of REAL Diabetes Study participants
| Data are mean ± SD or n (%) | Total N=81 |
Type 1 N=61 |
Type 2 N=20 |
p-value2 type 1 vs type 2 | Clinic recruit N=41 |
Social media or mailing recruit (SM/M) N=40 |
p-value2 clinic vs SM/M |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age (years) | 22.6 ± 3.5 | 22.5 ± 3.1 | 23.2 ± 4.5 | 0.98 | 21.4 ± 3.0 | 23.9 ± 3.6 | 0.002 |
| Gender – female | 51 (63) | 40 (66) | 11 (55) | 0.23 | 18 (44) | 33 (82) | 0.001 |
| Generation1 – 0 | 21 (26) | 12 (20) | 9 (45) | 0.06 | 14 (34) | 7 (18) | 0.13 |
| - 1 | 35 (43) | 27 (44) | 8 (40) | 18 (44) | 17 (42) | ||
| - 2 | 25 (31) | 22 (36) | 3 (15) | 9 (22) | 16 (40) | ||
| Race/Ethnicity – White | 8 (10) | 8 (13) | 0 | 0.20 | 1 (2) | 7 (18) | 0.11 |
| - Black | 8 (10) | 7 (11) | 1 (5) | 4 (10) | 4 (10) | ||
| - Hispanic/Latino | 63 (78) | 45 (74) | 18 (90) | 35 (85) | 28 (70) | ||
| - Other | 2 (2) | 1 (2) | 1 (5) | 1 (2) | 1 (2) | ||
| Hollingshead Index score (n=67) | 29.6 ± 13.1 | 31.0 ± 13.5 | 25.4 ± 11.1 | 0.13 | 25.1 ± 9.9 | 33.8 ± 14.4 | 0.02 |
| Neighborhood income ($K)* | 43.8 ± 16 | 45.3 ± 16.0 | 39.3 ± 15.3 | 0.08 | 39.1 ± 12.1 | 48.6 ± 18.0 | 0.02 |
| Neighborhood % below FPL* | 23.8 ± 11.3 | 22.3 ± 10.9 | 28.6 ± 11.5 | 0.03 | 25.2 ± 9.6 | 22.5 ± 12.8 | 0.10 |
| Recruitment – Clinic | 41 (51) | 33 (54) | 8 (40) | 0.31 | |||
| - Social media | 40 (49) | 28 (46) | 12 (60) | ||||
| Clinical | |||||||
| Diabetes type – T1D | 61 (75) | 33 (80) | 28 (70) | 0.31 | |||
| - T2D | 20 (25) | 8 (20) | 12 (30) | ||||
| Diabetes duration | 9.7 ± 5.8 | 11.1 ± 5.6 | 5.7 ± 4.2 | 0.0005 | 9.5 ± 5.6 | 10.0 ± 6.0 | 0.79 |
| A1C | 10.8 ± 1.9 | 10.6 ± 1.8 | 11.4 ± 2.1 | 0.12 | 10.6 ± 1.6 | 10.9 ± 2.1 | 0.72 |
| SDSCA glucose | 3.3 ± 2.7 | 3.6 ± 2.6 | 2.5 ± 2.8 | 0.10 | 3.8 ± 2.4 | 2.9 ± 2.9 | 0.11 |
| SDSCA medication | 5.9 ± 1.8 | 6.3 ± 1.3 | 4.6 ± 2.7 | 0.01 | 6.4 ± 1.4 | 5.3 ± 2.1 | 0.004 |
| SRBAI glucose | 15.0 ± 6.9 | 15.6 ± 6.9 | 13.1 ± 6.6 | 0.14 | 16.8 ± 6.5 | 13.1 ± 6.8 | 0.02 |
| SRBAI medication | 19.0 ± 6.3 | 20.1 ± 5.9 | 15.2 ± 6.4 | 0.01 | 19.7 ± 5.7 | 18.2 ± 6.9 | 0.41 |
| Treatment regimen | |||||||
| None | 3 (4) | 0 | 3 (15) | <0.0001 | 0 | 3 (8) | 0.08 |
| - Oral medication and/or non- insulin injectable only | 4 (5) | 0 | 4 (20) | 2 (5) | 2 (5) | ||
| - Insulin only | 63 (78) | 59 (97) | 4 (20) | 36 (88) | 27 (68) | ||
| - (Oral medication and/or non- insulin injectable) + insulin | 11 (14) | 2 (3) | 9 (45) | 3 (7) | 8 (20) | ||
| Among those on insulin | |||||||
| - Fixed regimen | 28 (38) | 16 (26) | 12 (92) | <0.0001 | 12 (31) | 16 (46) | 0.15 |
| - Intensive regimen | 42 (57) | 41 (67) | 1 (8) | 26 (67) | 16 (46) | ||
| - Unknown | 4 (5) | 4 (7) | 0 | 1 (3) | 3 (9) | ||
| - Insulin injections and/or pen | 65 (88) | 52 (85) | 13 (100) | 0.35 | 37 (95) | 28 (80) | 0.08 |
| - Insulin pump | 9 (12) | 9 (15) | 0 | 2 (5) | 7 (20) | ||
| Healthcare Utilization (over 12 months prior to randomization) | |||||||
| Number of routine diabetes visits (n=77) | 3.2 ± 1.8 | 3.2 ± 1.6 | 3.4 ± 2.4 | 0.89 | 3.6 ± 1.5 | 2.8 ± 2.1 | 0.02 |
| ≥ 2 visits with A1C recorded ≥3 months apart (n=77) | 49 (64) | 35 (61) | 14 (70) | 0.59 | 35 (85) | 14 (39) | 0.0001 |
| Proportion of participants reporting ≥1 diabetes-related hospitalization | 19 (23) | 15 (25) | 4 (20) | 0.77 | 9 (22) | 10 (25) | 0.80 |
| Psychosocial | |||||||
| Satisfaction with Life Scale | 20.5 ± 6.8 | 20.5 ± 6.8 | 20.6 ± 6.7 | 0.98 | 20.8 ± 6.7 | 20.3 ± 6.9 | 0.68 |
| Substance Abuse Screening (CAGE) | 0.5 ± 1.0 | 0.5 ± 1.0 | 0.6 ± 0.8 | 0.55 | 0.3 ± 0.7 | 0.7 ± 1.2 | 0.26 |
| Problem Activities in Diabetes-Short Form (range: 0–20) | 9.6 ± 5.7 | 9.2 ± 5.7 | 10.8 ± 5.6 | 0.25 | 8.6 ± 6.0 | 10.6 ± 5.3 | 0.12 |
| Audit of Diabetes-Dependent Quality of Life | −2.6 ± 1.7 | −2.7 ± 1.8 | −2.4 ± 1.5 | 0.59 | −2.3 ± 1.7 | −2.9 ± 1.7 | 0.10 |
| Diabetes Empowerment Scale | 31.4 ± 5.5 | 32.2 ± 5.2 | 29.1 ± 6.0 | 0.04 | 32.3 ± 4.7 | 30.5 ± 6.2 | 0.16 |
| Patient Health Questionnaire | 6.9 ± 5.0 | 7.0 ± 5.2 | 6.6 ± 4.5 | 0.75 | 6.3 ± 4.9 | 7.5 ± 5.0 | 0.22 |
| Diabetes Knowledge Questionnaire | 18.1 ± 3.2 | 18.4 ± 3.1 | 16.9 ± 3.5 | 0.08 | 17.2 ± 3.3 | 18.9 ± 3.0 | 0.01 |
| Participation, objective | −0.03 ± 0.2 | −0.0 ± 0.3 | −0.1 ± 0.2 | 0.14 | −0.1 ± 0.2 | 0.02 ± 0.3 | 0.10 |
| Participation, subjective | −0.03 ± 0.9 | 0.0 ± 0.9 | −0.2 ± 1.0 | 0.32 | −0.2 ± 0.8 | 0.14 ± 0.9 | 0.04 |
| Stressful Life Events | 5.0 ± 3.6 | 4.8 ± 3.8 | 5.4 ± 2.9 | 0.26 | 4.8 ± 3.4 | 5.1 ± 3.8 | 0.49 |
| Diabetes Problem Solving Inventory | 3.6 ± 0.6 | 3.7 ± 0.5 | 3.3 ± 0.9 | 0.04 | 3.6 ± 0.6 | 3.7 ± 0.7 | 0.32 |
0=Participant born outside United States; 1=Participant but neither parent born in United States; 2=At least one parent born in the United States;
Wilcoxon Rank-Sum Test or Fisher Exact Test;
Using 2010 Census tract data
3.3.1. Demographic characteristics
Participants’ age at baseline was 22.6 years old, and 63% of participants were female. Participants primarily identified as Hispanic/Latino (78%) with a large proportion being either immigrants (26%) or children of immigrants (43%). The majority of study participants (73%) lived with at least one family member from an older generation (a parent, aunt/uncle, or grandparent), and 16% of participants lived with their own child or children. Participants with T2D lived in neighborhoods with a higher proportion of households below the Federal poverty line than participants with T1D. Participants recruited at clinical sites were younger (21.4 vs. 23.9 years old), more likely to be male (54% vs. 18%), and lived in lower income neighborhoods than those recruited via SM/M.
3.3.2. Clinical characteristics
Overall, 75% of participants had T1D and 25% participants had T2D. The average duration of diabetes was 9.7 years, and average A1C was 10.8%. In comparing clinic-recruited versus SM/M-recruited participants, clinic-recruited participants had higher self-reported medication adherence (6.4 vs. 5.3 days/week) and stronger habit strength for blood glucose monitoring. Additionally, clinic-recruited participants had more routine diabetes care visits in the year prior to study enrollment (3.6 vs. 2.8 visits) and a greater proportion had at least two diabetes care visits at least three months apart at which an A1C value was documented (85% vs. 39%). In comparing participants with T1D and T2D, those with T1D had a longer duration of diabetes (11.1 vs. 5.7 years), greater self-reported medication adherence (6.3 vs. 4.6 days/week), and stronger habit strength for taking medication. Participants with T2D had a stronger family history of diabetes, among both first-degree relatives and extended family members. In comparing treatment regimens, while all participants with T1D took insulin, there was significant variability in regimens among participants with T2D, with 15% taking no medication; 20% taking oral and/or non-insulin injectable medications only; 20% taking insulin only; and 45% taking insulin and oral medications. Participants with T2D who were taking insulin were more likely to be on a fixed (versus flexible) regimen than their counterparts with T1D.
3.3.3. Psychosocial characteristics
Overall, participants had relatively poor psychosocial well-being. Most notably, 61.7% of participants had PAID-5 scores ≥8, the cutoff for clinically significant diabetes distress (41), and 27.2% had PHQ scores ≥10, the cutoff for potential major depressive disorder (46). In comparing participants with T1D versus T2D, participants with T1D had higher diabetes-related self-efficacy and greater diabetes-related problem-solving skills. In comparing clinic-recruited versus SM/M-recruited participants, those recruited from clinics had lower diabetes-related knowledge, and less satisfaction with everyday activity participation.
4. Discussion & Conclusion
The REAL Study aims to address the diabetes management needs of a particularly at-risk population that has been largely overlooked in both clinical and research contexts: low-SES, primarily minority young adults with diabetes. Among diabetes management interventions, those targeting young adults are vastly in the minority, and of the few interventions that have targeted this age group, even fewer have a high proportion of low-SES or racial/ethnic minority participants. Thus, the REAL Study, in examining the efficacy of a developmentally tailored intervention among low-SES and racial/ethnic minority young adults, addresses a critical unmet need in the field of diabetes management. Furthermore, it does so in a highly methodologically rigorous manner, in a randomized controlled trial utilizing blinding, adequate statistical power, population sampling, intervention fidelity monitoring, and adequate follow-up to ensure the validity of the study’s findings.
Recruiting and retaining low-SES and racial/ethnic minority participants into clinical research has been identified as one of the barriers to reducing health disparities amongst these populations. We utilized several strategies identified in previous research as facilitating successful recruitment and retention of ethnic minority and low-SES populations (48–51). These included offering in-home appointments to minimize logistical barriers to participation, ensuring language concordance among staff and participants, partnering with trusted clinical care providers and health systems, providing financial incentives for participation, and remaining in frequent contact with participants.
Remaining flexible with respect to recruitment strategies was also critical to the study’s success in recruitment. We encountered significant challenges at both of our clinical sites, which we were able to address through adopting alternative approaches to reach potential participants. At the children’s hospital, we initially approached potential participants in the waiting room with study information and enrollment materials. However, due to staffing limitations, recruiters did not have prior knowledge of participants’ clinical or demographic information and could not pre-screen participants, a particular challenge given that patients who met our inclusion criteria were a small minority of patients served at the clinic. Furthermore, we lacked sufficient staff to devote a full-time recruiter to this site. Because of these limitations, we had little success with in-person recruitment at the children’s hospital, with only two participants recruited in this manner ultimately enrolling in the study. Our adoption of a mass mailing was a much more effective strategy to reach potentially eligible participants from this site, ultimately yielding 11 enrolled participants.
In contrast to the children’s hospital, recruiters at the public hospital were integrated into the clinic workflow such that they could pre-screen potential participants, and patients who were eligible were approached in their exam rooms between provider visits. While this strategy was highly effective, we nonetheless encountered recruitment challenges when the public hospital experienced two major transitions in early 2015. First, the hospital was included in a County-wide transition to managed care, which disrupted the ability of outside providers to refer new patients to the clinic, and in some cases barred patients who were currently seen at the clinic from receiving care there. Second, the hospital began utilizing a new electronic medical record system, and in preparation, restricted the number of patients who could be scheduled for appointments. Although we continued in-person recruitment at the public hospital throughout the study’s recruitment period, significantly fewer patients were seen at the public hospital during this time due to these transitions. Thus, our adoption of social media advertising as a recruitment strategy was critical in ensuring the successful implementation of the study.
The participants in our study in general have poorer health and psychosocial well-being relative to those in other studies conducted among young adults with diabetes. Of particular note is the poor glycemic control evident in the study population, with an average A1C of 10.8%. This is significantly higher than recent epidemiological data showing an average A1C of 8.7% amongst 18–25 year olds with T1D (4), recent intervention studies amongst adolescents and young adults with T1D which ranged from 7.6% – 9.4% (11, 14, 15), and SEARCH study data reporting an average A1C of 8.5% among Hispanic-American youth with T2D, ages 15–19 years old (52). Our study’s inclusion criteria of having an A1C ≥8% accounts in part for this discrepancy. However, we did not turn away a large number of potential participants due to their glycemic control; only 30 of 241 individuals screened for inclusion were ineligible for this reason. We believe that the elevated A1C amongst our study population is in part an artifact of our inclusion criteria, but is also partly reflective of the challenges of managing diabetes in the context of low SES, which creates barriers to both accessing healthcare and carrying out disease management tasks in everyday life.
REAL Study participants’ psychosocial well-being was also lower than in comparable study populations. Amongst our participants, 27.2% had a PHQ score ≥10, indicating likely major depressive disorder. This is a significantly greater proportion than reported in other studies of adolescents and young adults with T1D, which ranged from 11–18% of participants (5, 6, 53). However, our rates are similar to those found by Browne and colleagues among a population of 18–39 year olds with T2D (6), and to those found in a study of adults with diabetes conducted in a free clinic setting characterized by low SES and low literacy (54). Similar trends were evident in diabetes-related quality of life, which was markedly lower than in studies amongst general populations of patients with diabetes (55, 56) and comparable to the free clinic population (54); and in life satisfaction, which was lower than that found in our previous research among low-SES, primarily minority young adults with T1D (11, 24). Thus, overall, the REAL study participants are indicative of a population that is vulnerable not only to poor physical outcomes, but demonstrates high levels of psychosocial distress.
While social media advertising and mass mailings (SM/M) were critical to our recruitment success, it is interesting to note that these strategies attracted a slightly different population than did our clinic-based recruitment. Participants recruited via SM/M were significantly older and more likely to be female than those recruited through clinical settings, and lived in higher-SES neighborhoods. The older age and higher income of participants recruited via SM/M may have contributed to our finding that they had significantly higher diabetes knowledge than those recruited from clinics. The difference in neighborhood SES is likely an artifact of our loosening of SES eligibility criteria during the stage of the study when SM/M participants were recruited; however, the reasons for age and gender discrepancies are less clear. Participants recruited from SM/M, despite having greater diabetes knowledge, also had poorer medication adherence and weaker habits for blood glucose self-monitoring than participants recruited at clinics. This is likely attributable to their more tenuous connections to medical care. Participants recruited at clinical sites typically had ongoing access to those clinics for needed medical care, whereas some participants recruited through SM/M may have had no regular source of medical care, had a shortage of needed diabetes care supplies, or had been receiving care from providers with less experience in managing T1D and youth-onset T2D than did the providers at our partnering clinical sites. Thus, in adopting a more broad-based recruitment strategy during our study, we ultimately recruited a study population with a greater need for support and services than if we had solely recruited through clinics.
The differences in study participants with T2D, as compared to those with T1D, are largely consistent with known attributes of T2D, such as having a stronger family history of disease than in T1D and being more prevalent in low-SES versus high-SES communities. Interestingly, participants with T2D generally had worse health indicators than those with T1D. They had slightly, though non-significantly, higher A1C (11.4% vs 10.6%; p=0.12), poorer medication adherence and habit strength for taking medications, and lower diabetes-related self-efficacy and problem-solving skills. While early-onset T2D is still poorly understood, it has been shown to be a significantly more aggressive disease than later-onset T2D, with a rapid onset of complications (57) and questionable utility of intensive treatment (58). This study provides further evidence of the precarious position of young adults with T2D, with respect to their current health and well-being as well as their risk of developing diabetes complications.
Conclusion
In summary, the REAL Study is addressing a significant unmet need in examining the efficacy of a diabetes management intervention tailored for an ethnically diverse, low-SES population of young adults with T1D and T2D. The study has successfully recruited and retained its target sample size through flexible implementation of a variety of strategies. In doing so, the REAL study has provided important information about the demographic, clinical, and psychosocial characteristics of an understudied population at particularly high risk of poor health and quality of life.
Acknowledgments
The authors gratefully acknowledge Alexandra Gonzalez, Alyssa Concha, Cindy Culp, Daniella Floríndez, Emily Friedberg, Eva Ortega, Jennie Lam, Jesus Diaz, Laura Cox, Laura Guzman, Maria Gonzalez, Nancy Dominguez, and Veronica Gomez for their contributions to this project.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (NIH/NIDDK #1K01 DK099202-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22(2):345–54. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Bell RA, Dabelea D, D’Agostino R, Jr, Imperatore G, Lawrence JM, et al. The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S99–101. doi: 10.2337/dc09-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendricks M, Monaghan M, Soutor S, Chen R, Holmes CS. A profile of self-care behaviors in emerging adults with type 1 diabetes. Diabetes Educ. 2013;39(2):195–203. doi: 10.1177/0145721713475840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current State of Type 1 Diabetes Treatment in the U.S.: Updated Data From the T1D Exchange Clinic Registry. Diabetes Care. 2015;38(6):971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 5.Johnson B, Elliott J, Scott A, Heller S, Eiser C. Medical and psychological outcomes for young adults with Type 1 diabetes: no improvement despite recent advances in diabetes care. Diabet Med. 2014;31(2):227–31. doi: 10.1111/dme.12305. [DOI] [PubMed] [Google Scholar]
- 6.Browne JL, Nefs G, Pouwer F, Speight J. Depression, anxiety and self-care behaviours of young adults with Type 2 diabetes: results from the International Diabetes Management and Impact for Long-term Empowerment and Success (MILES) Study. Diabet Med. 2015;32(1):133–40. doi: 10.1111/dme.12566. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein CM, Stockwell MS, Gallagher MP, Rosenthal SL, Soren K. Mental health issues in adolescents and young adults with type 1 diabetes: prevalence and impact on glycemic control. Clin Pediatr. 2013;52(1):10–5. doi: 10.1177/0009922812459950. [DOI] [PubMed] [Google Scholar]
- 8.Lancaster BM, Pfeffer B, McElligott M, Ferguson AT, Miller M, Wallace D, et al. Assessing treatment barriers in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2010;90(3):243–9. doi: 10.1016/j.diabres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Wibell L, Nystrom L, Ostman J, Arnqvist H, Blohme G, Lithner F, et al. Increased mortality in diabetes during the first 10 years of the disease. A population-based study (DISS) in Swedish adults 15–34 years old at diagnosis. J Intern Med. 2001;249(3):263–70. doi: 10.1046/j.1365-2796.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 10.Walders-Abramson N, Venditti EM, Ievers-Landis CE, Anderson B, El Ghormli L, Geffner M, et al. Relationships among stressful life events and physiological markers, treatment adherence, and psychosocial functioning among youth with type 2 diabetes. J Pediatr. 2014;165(3):504–8. e1. doi: 10.1016/j.jpeds.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequeira PA, Pyatak EA, Weigensberg MJ, Vigen CP, Wood JR, Ruelas V, et al. Let’s Empower and Prepare (LEAP): Evaluation of a Structured Transition Program for Young Adults With Type 1 Diabetes. Diabetes Care. 2015;38(8):1412–9. doi: 10.2337/dc14-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaic T, Mahon JL, Hramiak I, Byers N, Evans K, Robinson T, et al. Multicentre randomized controlled trial of structured transition on diabetes care management compared to standard diabetes care in adolescents and young adults with type 1 diabetes (Transition Trial) BMC Pediatr. 2013;13:163. doi: 10.1186/1471-2431-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Walleghem N, MacDonald CA, Dean HJ. Transition of care for young adults with type 1 and 2 diabetes. Pediatr Ann. 2012;41(5):e16–20. doi: 10.3928/00904481-20120426-20. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz JT, Laffel LM. Transitions in care: support group for young adults with Type 1 diabetes. Diabet Med. 2012;29(4):522–5. doi: 10.1111/j.1464-5491.2011.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markowitz JT, Cousineau T, Franko DL, Schultz AT, Trant M, Rodgers R, et al. Text messaging intervention for teens and young adults with diabetes. J Diabetes Sci Technol. 2014;8(5):1029–34. doi: 10.1177/1932296814540130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbeck KS, Shrewsbury VA, Harvey V, Mikler K, Donaghue KC, Craig ME, et al. A pilot randomized controlled trial of a post-discharge program to support emerging adults with type 1 diabetes mellitus transition from pediatric to adult care. Pediatr Diabetes. 2014;4(1):19–23. doi: 10.1111/pedi.12229. [DOI] [PubMed] [Google Scholar]
- 17.Clark F, Azen SP, Zemke R, Jackson J, Carlson M, Mandel D, et al. Occupational therapy for independent-living older adults. A randomized controlled trial. JAMA. 1997;278(16):1321–6. [PubMed] [Google Scholar]
- 18.Clark F, Jackson J, Carlson M, Chou CP, Cherry BJ, Jordan-Marsh M, et al. Effectiveness of a lifestyle intervention in promoting the well-being of independently living older people: results of the Well Elderly 2 Randomised Controlled Trial. J Epidemiol Community Health. 2012;66(9):782–90. doi: 10.1136/jech.2009.099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark F, Pyatak EA, Carlson M, Blanche EI, Vigen C, Hay J, et al. Implementing trials of complex interventions in community settings: the USC-Rancho Los Amigos Pressure Ulcer Prevention Study (PUPS) Clin Trials. 2014;11(2):218–29. doi: 10.1177/1740774514521904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNulty S. Lifestyle redesign: A successful tool for pain management. Painview. 2012;8(2):14–7. [Google Scholar]
- 21.Clark F, Azen SP, Carlson M, Mandel D, LaBree L, Hay J, et al. Embedding health-promoting changes into the daily lives of independent-living older adults: long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci. 2001;56(1):P60–3. doi: 10.1093/geronb/56.1.p60. [DOI] [PubMed] [Google Scholar]
- 22.Hay J, LaBree L, Luo R, Clark F, Carlson M, Mandel D, et al. Cost-effectiveness of preventive occupational therapy for independent-living older adults. J Am Geriatr Soc. 2002;50(8):1381–8. doi: 10.1046/j.1532-5415.2002.50359.x. [DOI] [PubMed] [Google Scholar]
- 23.Pyatak EA, Florindez D, Peters AL, Weigensberg MJ. “We are all gonna get diabetic these days”: the impact of a living legacy of type 2 diabetes on Hispanic young adults’ diabetes care. Diabetes Educ. 2014;40(5):648–58. doi: 10.1177/0145721714535994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyatak EA, Sequeira PA, Whittemore R, Vigen CP, Peters AL, Weigensberg MJ. Challenges contributing to disrupted transition from paediatric to adult diabetes care in young adults with Type 1 diabetes. Diabet Med. 2014;31(12):1615–24. doi: 10.1111/dme.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyatak EA, Carandang K, Davis S. Developing a manualized occupational therapy diabetes management intervention: Resilient, Empowered, Active Living With Diabetes. Otjr-Occupation Participation and Health. 2015;35(3):187–94. doi: 10.1177/1539449215584310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Occupational Therapy Association. AOTA Fact Sheet: The role of occupational therapy in chronic disease management. AOTA Press; Bethesda, MD: 2015. [Google Scholar]
- 28.Turner HA, Butler MJ. Direct and indirect effects of childhood adversity on depressive symptoms in young adults. Journal of Youth and Adolescence. 2003;32(2):89–103. [Google Scholar]
- 29.Turner RJ, Lloyd DA. Lifetime traumas and mental health: the significance of cumulative adversity. J Health Soc Behav. 1995;36(4):360–76. [PubMed] [Google Scholar]
- 30.Elhai JD, Layne CM, Steinberg AM, Brymer MJ, Briggs EC, Ostrowski SA, et al. Psychometric properties of the UCLA PTSD reaction index. part II: investigating factor structure findings in a national clinic-referred youth sample. J Trauma Stress. 2013;26(1):10–8. doi: 10.1002/jts.21755. [DOI] [PubMed] [Google Scholar]
- 31.Schilling EA, Aseltine RH, Gore S. The impact of cumulative childhood adversity on young adult mental health: Measures, models, and interpretations. Soc Sci Med. 2008;66(5):1140–51. doi: 10.1016/j.socscimed.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyatak EA, Sequeira P, Peters AL, Montoya L, Weigensberg MJ. Disclosure of psychosocial stressors affecting diabetes care among uninsured young adults with Type 1 diabetes. Diabet Med. 2013;30(9):1140–4. doi: 10.1111/dme.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36(12):1111–9. doi: 10.1016/j.addbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56(1):44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Mora C, Rodriguez-Oliva MS, Fernandez-Riejos P, Mateo J, Polo-Padillo J, Goberna R, et al. Evaluation of two HbA1c point-of-care analyzers. Clin Chem Lab Med. 2011;49(4):653–7. doi: 10.1515/CCLM.2011.101. [DOI] [PubMed] [Google Scholar]
- 36.Toobert DJ, Hampson S, Glasgow RE. The Summary of Diabetes Self-Care Activities Measure: Results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 37.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8(1–2):79–91. doi: 10.1023/a:1026485130100. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF) Diabetes Care. 2003;26(5):1641–2. doi: 10.2337/diacare.26.5.1641-a. [DOI] [PubMed] [Google Scholar]
- 39.Garcia AA, Villagomez ET, Brown SA, Kouzekanani K, Hanis CL. The Starr County Diabetes Education Study: development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care. 2001;24(1):16–21. doi: 10.2337/diacare.24.1.16. [DOI] [PubMed] [Google Scholar]
- 40.Glasgow RE, Fisher L, Skaff M, Mullan J, Toobert DJ. Problem Solving and Diabetes Self-Management: Investigation in a large, multiracial sample. Diabetes Care. 2007;30(1):33–7. doi: 10.2337/dc06-1390. [DOI] [PubMed] [Google Scholar]
- 41.McGuire BE, Morrison TG, Hermanns N, Skovlund S, Eldrup E, Gagliardino J, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53(1):66–9. doi: 10.1007/s00125-009-1559-5. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Brown M, Dijkers MPJM, Gordor WA, Ashman T, Charatz H, Cheng ZF. Participation objective, participation subjective - A measure of participation combining outsider and insider perspectives. J Head Trauma Rehabil. 2004;19(6):459–81. doi: 10.1097/00001199-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Gardner B, Abraham C, Lally P, de Bruijn GJ. Towards parsimony in habit measurement: Testing the convergent and predictive validity of an automaticity subscale of the Self-Report Habit Index. Int J Behav Nutr Phys Act. 2012:9. doi: 10.1186/1479-5868-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 46.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ : Canadian Medical Association Journal. 2012;184(3):E191–E6. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- 48.Paskett ED, Reeves KW, McLaughlin JM, Katz ML, McAlearney AS, Ruffin MT, et al. Recruitment of minority and underserved populations in the United States: The centers for population health and health disparities experience. Contemp Clin Trials. 2008;29(6):847–61. doi: 10.1016/j.cct.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholson LM, Schwirian PM, Klein EG, Skybo T, Murray-Johnson L, Eneli I, et al. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp Clin Trials. 2011;32(3):353–62. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinton L, Carter K, Reed RB, Beckett L, Lara E, DeCarli C, et al. Recruitment of a Community-based Cohort for Research on Diversity and Risk of Dementia. Alzheimer Dis Assoc Disord. 2010;24(3):234–41. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence JM, Mayer-Davis EJ, Reynolds K, Beyer J, Pettitt DJ, D’Agostino RB, Jr, et al. Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S123–32. doi: 10.2337/dc09-S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachle C, Lange K, Stahl-Pehe A, Castillo K, Holl RW, Giani G, et al. Associations between HbA1c and depressive symptoms in young adults with early-onset type 1 diabetes. Psychoneuroendocrinology. 2015;55:48–58. doi: 10.1016/j.psyneuen.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Bowser DM, Utz S, Glick D, Harmon R, Rovnyak V. The Relationship Between Diabetes Mellitus, Depression, and Missed Appointments in a Low-Income Uninsured Population. The Diabetes Educator. 2009;35(6):966–77. doi: 10.1177/0145721709345164. [DOI] [PubMed] [Google Scholar]
- 55.Iversen MM, Espehaug B, Rokne B, Haugstvedt A, Graue M. Psychometric properties of the Norwegian version of the Audit of Diabetes-Dependent Quality of Life. Qual Life Res. 2013;22(10):2809–12. doi: 10.1007/s11136-013-0413-x. [DOI] [PubMed] [Google Scholar]
- 56.Ostini R, Dower J, Donald M. The Audit of Diabetes-Dependent Quality of Life 19 (ADDQoL): feasibility, reliability and validity in a population-based sample of Australian adults. Qual Life Res. 2012;21(8):1471–7. doi: 10.1007/s11136-011-0043-0. [DOI] [PubMed] [Google Scholar]
- 57.Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Mayo Clin Proc. 2014;89(6):806–16. doi: 10.1016/j.mayocp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med. 2012;29(4):453–63. doi: 10.1111/j.1464-5491.2011.03542.x. [DOI] [PubMed] [Google Scholar]