Figure 1. Steps in the development in PCP from RGCs to E1 cells, in the brain ventricular epithelia.
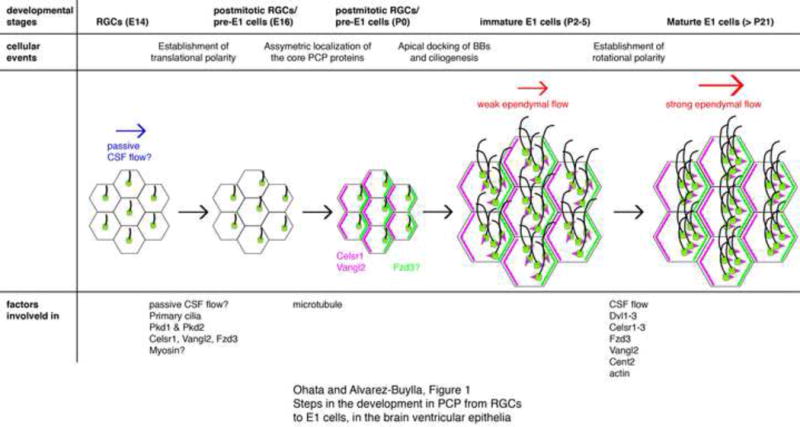
At embryonic day (E) 14, primary cilia in RGCs are located near the center on the apical surface. By E16, the primary cilia become asymmetrically displaced towards the downstream of the CSF flow. Initial passive CSF flow in the embryonic ventricles (blue arrow) could instruct this asymmetric localization of RGCs’ primary cilia via the mechanosensory proteins. Pkd1 and Pkd2 have been found in RGCs’ primary cilia and partially contribute to PCP. Ablation of celsr1, vangl2, or fzd3 also affects this translational polarity in RGCs. Myosins are likely involved in the translational polarization in RGCs. The core PCP proteins Celsr1 and Vangl2 start to localize asymmetrically in the apical area of RGCs by P0. Fzd3 also localizes asymmetrically in immature E1 cells at P5 but its localization in P0 RGCs has not been reported. Microtubules are important for the asymmetric localization of Vangl2 and Celsr1 in P2 RGCs. Newly generated BBs dock to the apical area of immature E1 cells and motile cilia are formed around P2–5. At this stage, rotational polarity indicated by the positioning of basal feet (magenta triangles) is random and the ependymal flow is weak (smaller red arrow). Rotational polarity becomes aligned with the direction of CSF flow as the ependymal layer matures; the rotational polarity is further refined and reinforced (bigger red arrow). The model suggests that CSF flow, together with Dvl1–3, Celsr1–3, Fzd3, Vangl2, and Cent2, are involved in the establishment of rotational polarity.
