Table 3. Binding Constant, Free Energy, Enthalpy, and Entropy of Binding Derived from ITC Measurements for M2TMUdorn/72.
| liganda | Kdb | ΔGc,d | ΔHc,e | –TΔSc,f |
|---|---|---|---|---|
| 1 | 2.17 ± 0.52 | –7.77 ± 0.14 | –6.66 ± 0.50 | –1.11 ± 0.52 |
| 2 | 0.51 ± 0.26 | –8.64 ± 0.30 | –7.60 ± 0.28 | –1.04 ± 0.41 |
| 2-Rg | 0.32 ± 0.16 | –8.97 ± 0.26 | –7.54 ± 0.34 | –1.42 ± 0.43 |
| 2-Sg | 0.34 ± 0.12 | –8.88 ± 0.21 | –7.73 ± 0.28 | –1.15 ± 0.35 |
| 3 | 0.13 ± 0.12 | –9.30 ± 0.43 | –4.19 ± 0.28 | –5.12 ± 0.51 |
Binding constant Kd in μM calculated from measured Ka in M–1 by Kd = 1/Ka × 10–6 and error in Kd in μM determined by Kd, error = (Ka, error/Ka2) × 10–6 (ITC measurements were performed in triplicate for each ligand to calculate means and standard deviations).
In kcal mol–1.
Free energy of binding
computed
from Kd by ΔG =
−RT ln(Kdref/Kd) with Kdref = 1 M and T = 300 K and
error in ΔG determined according to 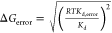 with T = 300 K.
with T = 300 K.
Enthalpy of binding and error in the enthalpy of binding calculated from measured binding enthalpy and measured error by ΔH = ΔHmeasured (T/Tmeasured) with T = 300 K and the temperature at which the ITC measurements were performed Tmeasured = 293.15 K.
Entropy of
binding calculated by
ΔS = (−ΔG + ΔH)/T and error in ΔS computed by the equation 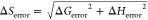 .
.
The purity of each enantiomer used was 90% for 2-R and 95% for 2-S; the enantiomeric excess (ee) of both 2-R and 2-S is 99% (Mosher’s method); the purity of compound 3 used was >99%.
