Figure 2.
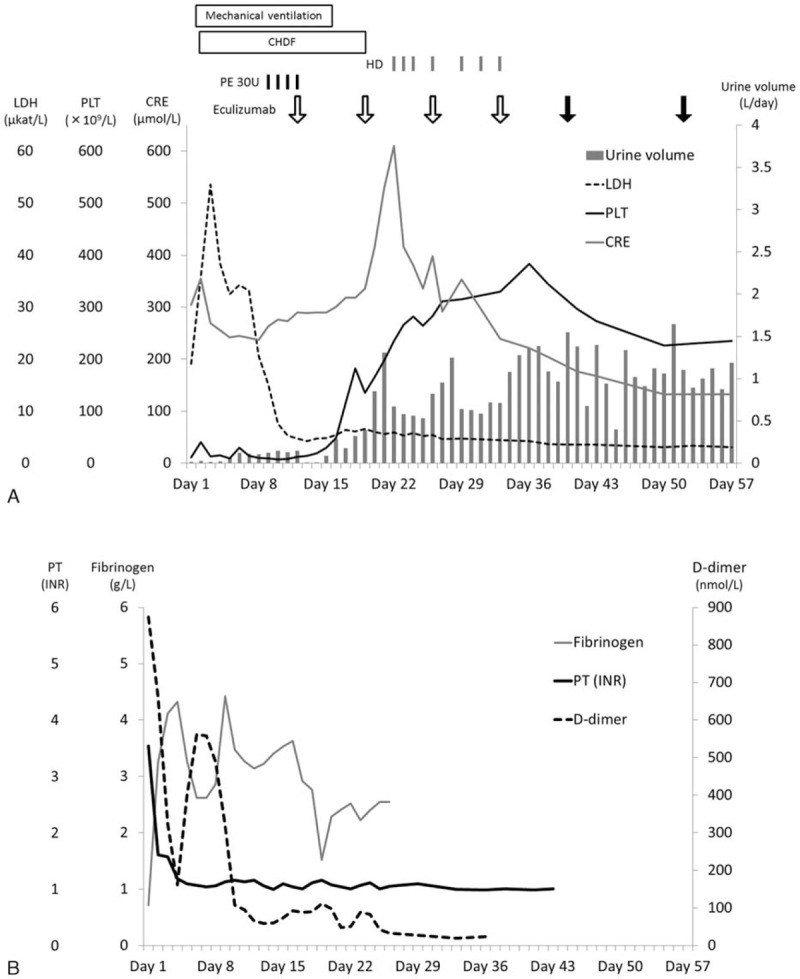
Summary of clinical values of thrombotic microangiopathy (A) and disseminated intravascular coagulation (B). (A) At admission, the patient exhibited symptoms of thrombotic microangiopathy (thrombocytopenia and elevated serum levels of lactate dehydrogenase and creatinine). We clinically diagnosed atypical hemolytic uremic syndrome and performed plasma exchange on day 9. Plasma exchange (black bar) was initiated owing to decreased platelet count (black line) and renal dysfunction (serum creatinine, gray line; urine volume, gray column), which persisted despite decreased lactate dehydrogenase levels (dotted line). We administrated 900 mg of eculizumab (open arrow) on day 12. After eculizumab treatment, the thrombocytopenia and oliguria resolved, although creatinine levels transiently elevated due to transition from continuous hemodiafiltration to intermittent hemodialysis. On day 19, mechanical ventilation was discontinued. Because of an increase of urine volume, continuous hemodiafiltration was discontinued on day 19, and intermitted hemodialysis was performed from day 22 to day 33. The eculizumab dosage was increased to 1200 mg (closed arrow) after the fifth eculizumab administration. Every value of thrombotic microangiopathy remained in remission. (B) The coagulation tests indicated disseminated intravascular coagulation (prolonged prothrombin time, reduced levels of fibrinogen, and elevated levels of D-dimer). The abnormalities indicative of DIC (prothrombin time, black line; fibrinogen, gray line) had resolved except for elevated D-dimer levels (dotted line) on day 8. D-dimer levels normalized after administration of eculizumab. APTT = activated partial thromboplastin time, CHDF = continuous hemodiafiltration, CRE = creatinine, DIC = disseminated intravascular coagulation, HD = hemodialysis, LDH = lactate dehydrogenase, PE = plasma exchange, PLT = platelet count, PT (INR) = prothrombin time international normalized ratio.
