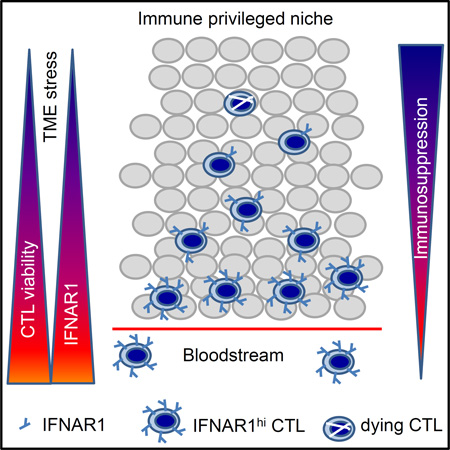
Abstract
Refractoriness of solid tumors including colorectal cancers (CRC) to immunotherapies is attributed to the immunosuppressive tumor microenvironment that protects malignant cells from cytotoxic T lymphocytes (CTL). We found that downregulation of the type I interferon receptor chain IFNAR1 occurs in human CRC and mouse models of CRC. Downregulation of IFNAR1 in tumor stroma stimulated CRC development and growth, played a key role in formation of the immune privileged niche and predicted poor prognosis in human CRC patients. Genetic stabilization of IFNAR1 improved CTL survival and increased the efficacy of the chimeric antigen receptor T cell transfer and PD-1 inhibition. Likewise, pharmacologic stabilization of IFNAR1 suppressed tumor growth providing the rationale for upregulating IFNAR1 to improve anti-cancer therapies.
Keywords: cytotoxic T cells, tumor microenvironment, interferon, receptor, IFNAR1, immunosuppression, colorectal cancer
Graphical Abstract
Katlinski et al. show reduced type I interferon receptor chain IFNAR1 in colorectal cancer (CRC) stroma, which is important in forming the immune privileged niche to support CRC development and growth. Stabilization of IFNAR1 improves cytotoxic T lymphocyte survival and suppresses tumor growth.
INTRODUCTION
The presence of tumor-specific T cells in the blood of cancer patients, the “Hellstrom paradox” (Hellstrom et al., 1968), suggests that although developing cancers are able to induce a comprehensive immune response, tumors can progress by hindering anti-tumor effector cells (Klemm and Joyce, 2015; Quail and Joyce, 2013). However, recent success in cancer immunotherapy indicates that augmentation of the immune response can improve prognosis. As such, current approaches to cancer immunotherapy focus on increasing either tumor antigen presentation (via vaccines) or the number of tumor-specific CD8+ CTL (via chimeric antigen receptors (CAR) therapy and other types of adoptive transfer) or enhancing CTL activities (via checkpoint inhibition; reviewed in (Rosenberg and Restifo, 2015; Sharma and Allison, 2015)).
Regrettably, the majority of patients with solid tumors including colorectal cancers (CRC) are refractory to these treatments (Brahmer et al., 2012; Gilham et al., 2012; Topalian et al., 2012). Solid tumors evade anti-cancer immune control by establishing immune-privileged niches within the tumor microenvironment (TME). Diverse cellular and acellular (e.g. deficit of oxygen and nutrients) TME elements reduce proliferation, viability or activity of intra-tumoral CTL thereby inhibiting their anti-tumor effector function (Fearon, 2014; Joyce and Fearon, 2015; Zhou et al., 2014). Indeed, the apparent exclusion of CTL from CRC is associated with a poor prognosis (Chiba et al., 2004; Galon et al., 2006; Naito et al., 1998) while, conversely, increased accumulation of CTL within tumors is associated with a favorable outcome (Talmadge et al., 2007). Delineating the mechanisms that prevent CTL accumulation within the TME remains a major challenge in understanding the immunosuppressive properties of the TME and increase efficacy of immunotherapies (Joyce and Fearon, 2015).
Studies modeling sarcomas and melanomas in mice lacking the IFNAR1 chain of Type I interferons (IFN) receptor suggest that endogenous IFN contribute to anti-tumor immunity via stimulating specific CD8α+ lineage dendritic cells (DCs) to cross-present antigen to CTL (Diamond et al., 2011; Fuertes et al., 2011; Hildner et al., 2008). IFN also provide a “third signal” to stimulate the clonal expansion of CD8+ T cells (Aichele et al., 2006; Curtsinger et al., 2005; Hervas-Stubbs et al., 2010) and increase viability of activated anti-viral CD8+ T lymphocytes (Crouse et al., 2014; Kolumam et al., 2005; Wang et al., 2012; Xu et al., 2014) and tumor-specific CTL (Hiroishi et al., 2000). These reports are consistent with the long known anti-tumorigenic effects of IFN (Platanias, 2005; Trinchieri, 2010; Zitvogel et al., 2015). Nevertheless, given that tumorigenesis readily proceeds in IFNAR1-competent mice and humans, it is apparent that cancers manage to overcome the effects of endogenous IFN through a yet poorly understood mechanism.
Cell surface IFNAR1 levels are critical for all IFN effects (Fuchs, 2013; Uze et al., 2007). These levels are controlled by IFNAR1 ubiquitination and degradation facilitated by the SCF-βTrcp E3 ligase, which binds to IFNAR1 phosphorylated on Ser535 (Ser526 in mouse IFNAR1; (Kumar et al., 2003). Phosphorylation of these serine residues can be triggered in vitro by stimuli characteristic for tumor microenvironment such as unfolded protein response (Liu et al., 2009), oxygen or nutrient deficit (Bhattacharya et al., 2013), vascular endothelial growth factor (Zheng et al., 2011) and inflammatory cytokines (Huangfu et al., 2012). Here we aimed to characterize the status of IFNAR1 and IFN signaling in CRC tumors and to determine the importance of IFNAR1 downregulation in establishing the intra-tumoral immune privileged niche.
RESULTS
IFNAR1 levels and signaling are reduced in the TME
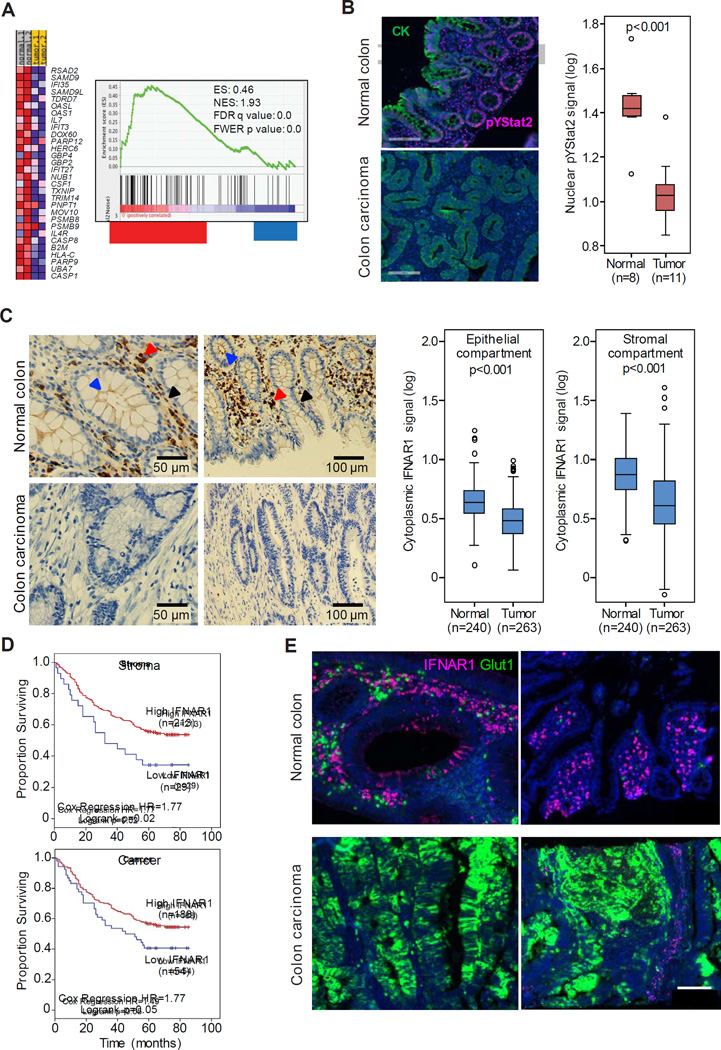
Global expression profiling within hypoxic areas of transplanted tumors revealed a decrease in expression of the immune response genes (Marotta et al., 2011). We also noted a suppression of the IFN signaling signature in hypoxic tumor areas characterized by TME stress (Figure S1A–S1B). Importantly, mining of datasets from patients with CRC (Rohr et al., 2013) revealed a decrease in IFN-induced gene expression in tumors compared to benign colorectal tissues from the same patients (Figure 1A). In addition, compared to normal colorectal tissues, tumors exhibited markedly decreased levels of nuclear phosphorylated STAT2 (Figure 1B), which is a downstream effector of IFN signaling (Platanias, 2005). These results suggest that IFN signaling is inhibited in human CRC tumors.
Figure 1. IFNAR1 levels and signaling are reduced in colorectal adenocarcinomas.
(A) Heat map and Gene Set Enrichment Analysis (GSEA) of IFN signaling pathway genes of the transcriptome profiles of human normal colon and matched CRC tissues (from (Rohr et al., 2013).
(B) Representative immunofluorescent analysis of phospho-Tyr-STAT2 (red) and pancytokeratin (CK, green) in normal and malignant colon tissues counterstained with DAPI (blue). Box plot showing nuclear pTyr-Stat2 levels in representative normal (n=8) and cancer (n=11) cases indicates median (dark line), 25–75% range (box), minimum and maximum values (whiskers) and individual scatter plot values (circles) overlaying the box plot.
(C) Representative chromogen immunohistochemistry analysis of IFNAR1 in normal and malignant colon. Arrows point to IFNAR1-positive fibroblasts (black), epithelial cells (blue) and immune cells (red). Box plots (as in B) show cytoplasmic IFNAR1 expression levels in the epithelial (left) or stromal (right) compartments of malignant colon and adjacent normal tissue
(D) Kaplan-Meier plot of cases of colorectal adenocarcinomas based on levels of cytoplasmic IFNAR1
(E) IFNAR1 (red) and GLUT1 (green)-positive cells in normal and malignant human colon tissues. See also Figure S1.
TME-associated stress stimuli such as nutrient/oxygen deficit can cause a loss of IFNAR1 protein in vitro (Bhattacharya et al., 2013). Although comparable IFNAR1 mRNA expression was reported in CRC and normal colorectal tissues (Rohr et al., 2013), we noted dramatic differences in IFNAR1 protein levels. IFNAR1 was detected in all normal human colon cell types including epithelial cells (especially at their apical surface), stromal fibroblasts and infiltrating immune cells. However, all cell types within human colorectal adenocarcinomas exhibited partial or complete loss of IFNAR1 (Figure 1C and S1C). For these samples, IFNAR1 levels in cancer cell compartment and in the stromal compartment positively correlated (r=0.700, p<0.001; n=263). Importantly, downregulation of IFNAR1 in either stromal or cancer cell compartments of human CRC tumors were associated with poor prognosis (Figure 1D). Furthermore, whereas many cells expressed high levels of IFNAR1 in normal human colon, those few IFNAR1-positive cells found in colon carcinomas were spatially segregated from the tumor areas, which were positive for GLUT1, a marker of TME stress (Figure 1E and S1D). These data collectively suggest that TME conditions in human CRC prompt IFNAR1 downregulation and suppress IFN signaling.
Downregulation of IFNAR1 in the stromal compartment stimulates colorectal tumorigenesis
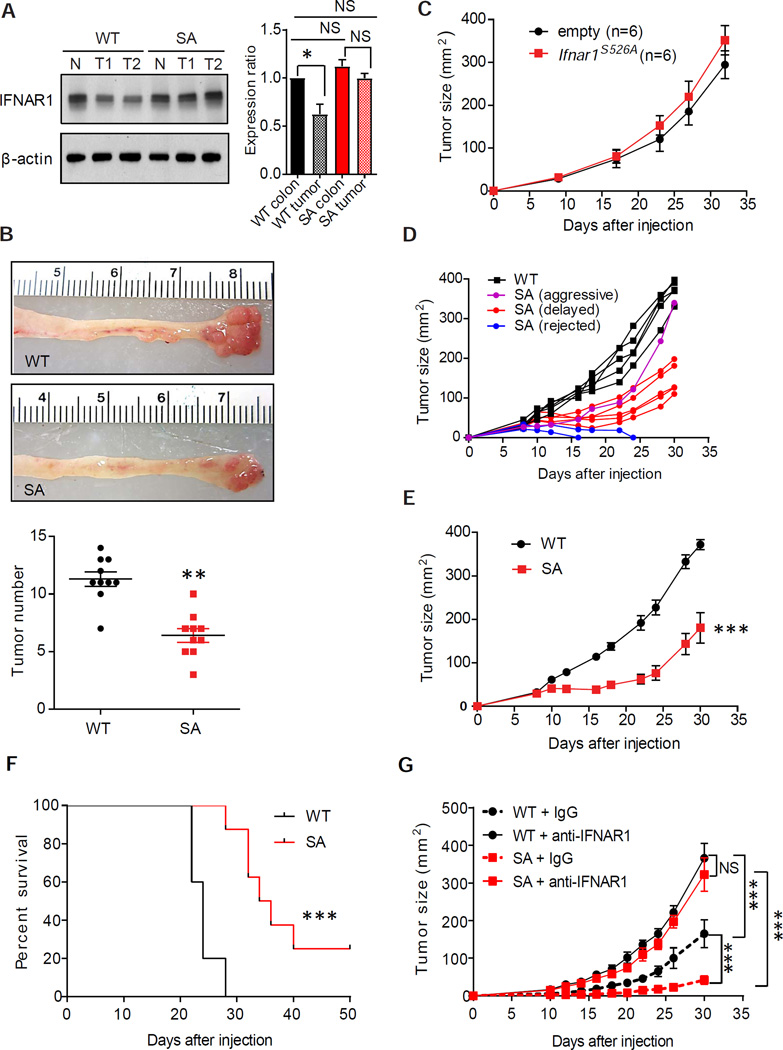
Guided by these data in human patients, we sought to determine the role of partial loss of IFNAR1 using murine CRC models. Notably, downregulation of IFNAR1 protein observed in human CRC (Figure 1C) was faithfully recapitulated in the mouse model of inflammatory colorectal carcinogenesis induced by treatment with azoxymethane and dextran sodium sulfate (AOM-DSS). The observed decrease in levels of IFNAR1 protein (Figures 2A and S2A) but not Ifnar1 mRNA (Figure S2B) in AOM-DSS-induced colorectal tumors suggested an increased IFNAR1 degradation within tumors. Therefore, we next used Ifnar1S526A mice (henceforth “SA”) previously shown to be deficient in IFNAR1 ubiquitination and degradation (Bhattacharya et al., 2014). SA mice treated with AOM-DSS sustained high levels of IFNAR1 protein (Figure 2A and S2A) and mRNA for IFN-stimulated and inflammatory genes (Figure S2C) relative to wild type (WT) mice. Importantly, AOM-DSS treatment induced fewer tumors in SA mice (Figure 2B) indicating that downregulation of IFNAR1 contributes to efficient colorectal tumorigenesis.
Figure 2. Downregulation of IFNAR1 in the stromal compartment stimulates colorectal tumorigenesis.
(A) Immunoblot analysis of IFNAR1 immunoprecipitated from the whole tissue lysates prepared from normal colon or AOM-DSS-induced tumors from WT and SA mice. The IFNAR1/β-actin (loading control) signal relative ratios calculated from n=6 for each group (WT colon taken as 1.0 and shown as Mean±SD) are depicted on the right. Henceforth asterisks: * p<0.05; ** p<0.01; *** p<0.001.
(B) Representative images and quantification of colorectal tumors in mice of indicated genotypes at day 70 after treatment with AOM-DSS.
(C) Growth of MC38mRFP cells that received GFP or IFNAR1S526A-GFP constructs after s.c. injection into WT mice (Mean±SEM, n=6).
(D) Subcutaneous growth of individual MC38 tumors in WT and SA mice.
(E) A representative experiment demonstrating average size of MC38 tumors growing in WT (n=5) and SA (n=8) mice (Mean±SEM).
(F) Kaplan-Meier analysis of survival of MC38 tumor-bearing WT and SA mice from E.
(G) Effect of anti-IFNAR1 neutralizing antibodies on MC38 tumor growth in WT and SA mice (Mean±SEM, n=5–6 for each of 2 experiments). See also Figure S2.
In a transplantation model, tumors formed in WT mice by MC38 colon adenocarcinoma cells expressed lower levels of IFNAR1 compared with these cells cultured in vitro (Figure S2D), demonstrating that the MC38 tumor model re-capitulates the IFNAR1 loss observed in human CRC. To determine the importance of IFNAR1 downregulation in malignant cell compartment, we next aimed to restore IFNAR1 levels in MC38 cancer cells. Previous studies in fibrosarcomas and mammary adenocarcinomas demonstrated a tumor suppressive effect of the IFN signaling in malignant cells (Bidwell et al., 2012; Sistigu et al., 2014). We also reported that expression of the Ifnar1S526A allele in Ifnar1-null mouse melanoma cell line delays growth of these tumors (Katlinskaya et al., 2016). However, expression of the Ifnar1S526A allele in MC38mRFP cells did not affect their ability to form tumors in WT mice (Figure 2C, S2E–F), indicating that cell-autonomous anti-tumorigenic effects of IFNAR1 expression and IFN signaling might be cell- or tumor type-specific.
To determine the role of IFNAR1 downregulation in the stromal compartment, we inoculated WT or SA mice with MC38 cells (Figure S2G). While WT mice readily supported tumor growth, very few of MC38 tumors grew aggressively in SA mice (Figure 2D). Notably, most of these tumors were either rejected or exhibited a delayed growth (Figures 2D–E and S2H) resulting in a prolonged survival (Figure 2F) in SA mice suggesting an important role of downregulation of stromal IFNAR1 in tumorigenesis. Indeed, injection of IFNAR1-neutralizing antibodies further stimulated MC38 tumor growth in WT mice and dramatically rescued tumor growth in SA hosts (Figure 2G). These results indicate that efficient tumor growth requires downregulation of IFNAR1 levels primarily in the non-malignant cells.
Alterations of gene expression associated with IFNAR1 downregulation correlate with local immunosuppression and poor prognosis in CRC patients
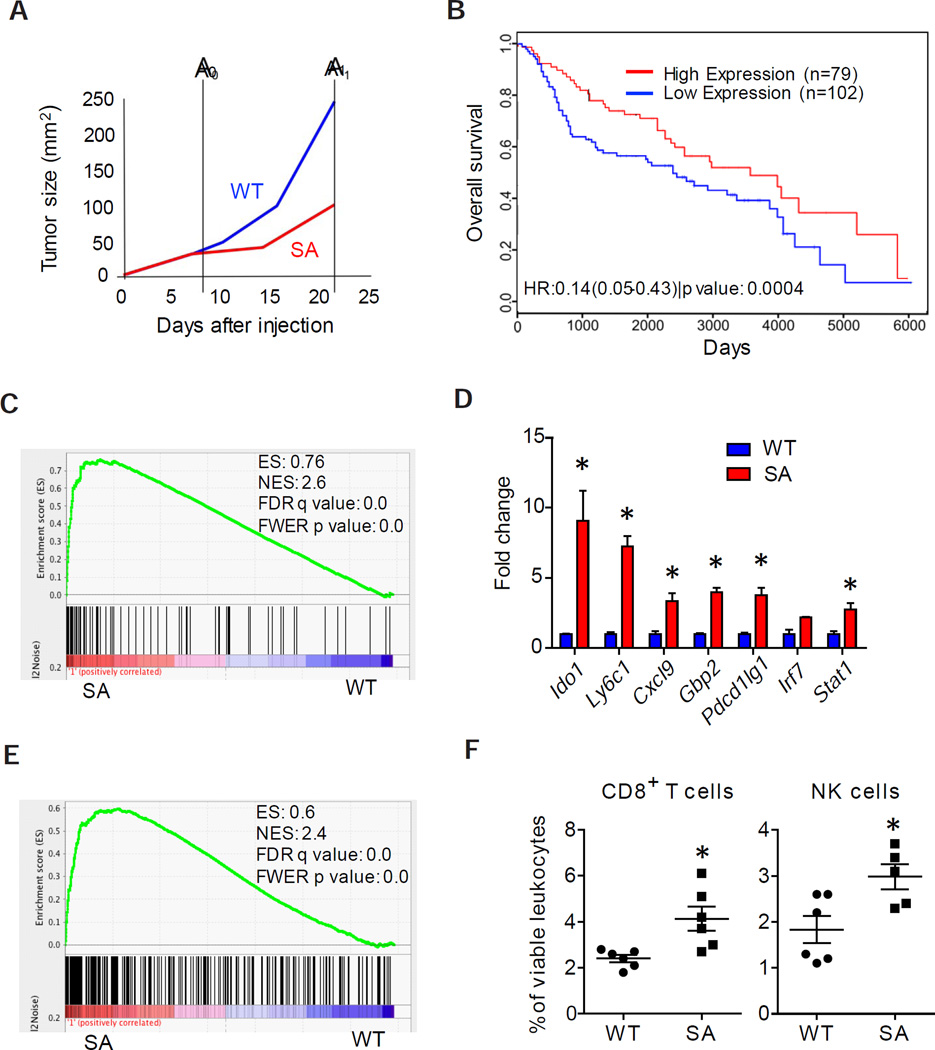
We next profiled the gene expression associated with IFNAR1 downregulation (in tumors with WT stroma) or stabilized IFNAR1 (in tumors with SA stroma). Notably, at an early time point after MC38 transplantation (A0, Figure 3A), the stromal compartments from tumors of comparable size that arose in WT or SA mice already differed in their gene expression patterns (Table S1). While most of differentially expressed genes (e.g. Irf7, Ifit2, Mx2, Usp18, etc) are well known to be induced by IFN, others (Clec7a, Sdc3) have not been previously reported as bona fide IFN-regulated genes in global expression studies (Mostafavi et al., 2016; Rusinova et al., 2013) suggesting that downregulation of IFNAR1 in the context of growing tumor may elicit a more complex response than merely an IFN signaling suppression. Specifically, the status of a set of 30 genes whose expression was increased in the stroma of early mouse SA tumors compared with WT ones (Table S1) was associated with impaired tumor progression in SA mice (Figures 2D–E, S2H).
Figure 3. Alterations of gene expression associated with IFNAR1 downregulation correlate with local immunosuppression and with a poor prognosis in CRC patients.
(A) Schematic representation of MC38 tumor growth in WT and SA mice. Time points of harvesting tumors of comparable (A0) and disparate (A1) size are indicated.
(B) Survival of adjusted for the stage CRC patients (GSE41258) harboring expression pattern of 30 selected genes similar to MC38 that grew either in WT (blue) or in SA (red) mice
(C) GSEA results of IFNα/β signaling pathway in tumors harvested at day 9 (time point A0) and used for RNA isolation and microarray analysis.
(D) qPCR analysis of indicated genes expressed in WT and SA tumors.
(E) GSEA results for the immune system process in tumors harvested at A0.
(F) Percent of NK and CD8+ T cells (relative to CD45+ cells) infiltrating MC38 tumors in WT and SA mice.
Data are shown as Mean±SEM from 5–6 tumors.
More importantly, this gene expression profile was also predictive of a better prognosis in two separate stage-adjusted cohorts of human CRC patients (Figure 3B and S3A). Furthermore, dramatic suppression of the IFN-induced genes (Figure 3C and 3D) correlated with subsequent aggressive tumor growth (Figure 2D) was seen in early WT (but not SA) tumors. In all, these data suggest that IFNAR1 downregulation and the ensuing alterations in gene expression contribute to CRC progression and appear to be predictive of disease outcome in human CRC patients.
Additional comparison of WT and SA gene expression patterns revealed a suppressed immune pathway in early WT tumors (Figure 3D–E) pointing to the immune system as a putative target of IFNAR1 downregulation. At later time points, when tumors in WT mice became larger than tumors in SA mice (A1, Figure 3A), we noted a similar suppression of IFN signaling and immune genes signature in WT tumors (Figures S3B–C). These results indicate that decreased IFNAR1 levels in the stromal compartment may determine both the immunosuppressive capacity and growth potential of the tumor.
Prompted by gene expression data we compared the levels of immune cells in WT and SA mice burdened with MC38 tumors. Compared to SA mice, spleens from WT mice contained somewhat greater overall levels of CD11b+Ly6G+ cells; however, we did not detect significant differences in the frequencies of splenic NK cells or T cells (Figure S3D) that would be characteristic of generalized immunosuppression in tumor-bearing WT animals. Conversely, analysis of tumor-infiltrating leukocyte subsets revealed significantly reduced frequencies of CD8+ T cells, NK and Ly6ChiLy6G− cells in tumors from WT animals compared to tumors from SA mice (Figure 3F and S3E). This result indicates that downregulation of IFNAR1 within WT tumors is associated with a localized intra-tumoral immunosuppression, resulting in reduced CTL accumulation within the TME.
Downregulation of IFNAR1 in cytotoxic T lymphocytes contributes to development of the immunosuppressive tumor microenvironment in CRC
Previous studies of sarcomas and melanomas grown in WT or Ifnar1 knockout mice suggested a critical role of IFN in the ability of specific CD8α+ lineage dendritic cells (DCs) to cross-present antigen to the CD8+ cytotoxic lymphocytes and highlighted the critical role of these DCs in anti-tumor immune protection (Diamond et al., 2011; Fuertes et al., 2011; Hildner et al., 2008). We did not observe changes in the overall frequency of intra-tumoral CD11b+ CD11c+ MHCII+ CD103− or CD11c+ MHCII+ CD8α+ or CD11c+ MHCII+ CD103+ DCs between MC38 tumors that grew in WT and SA mice (Figure S3E). DCs isolated from WT or SA mice exhibited a similar efficacy in direct antigen presentation (Figure S4A) as well as in cross-presentation of tumor antigens (Figure S4B) indicating that reduced tumorigenesis in SA mice cannot be readily explained by an increased antigen presentation capacity.
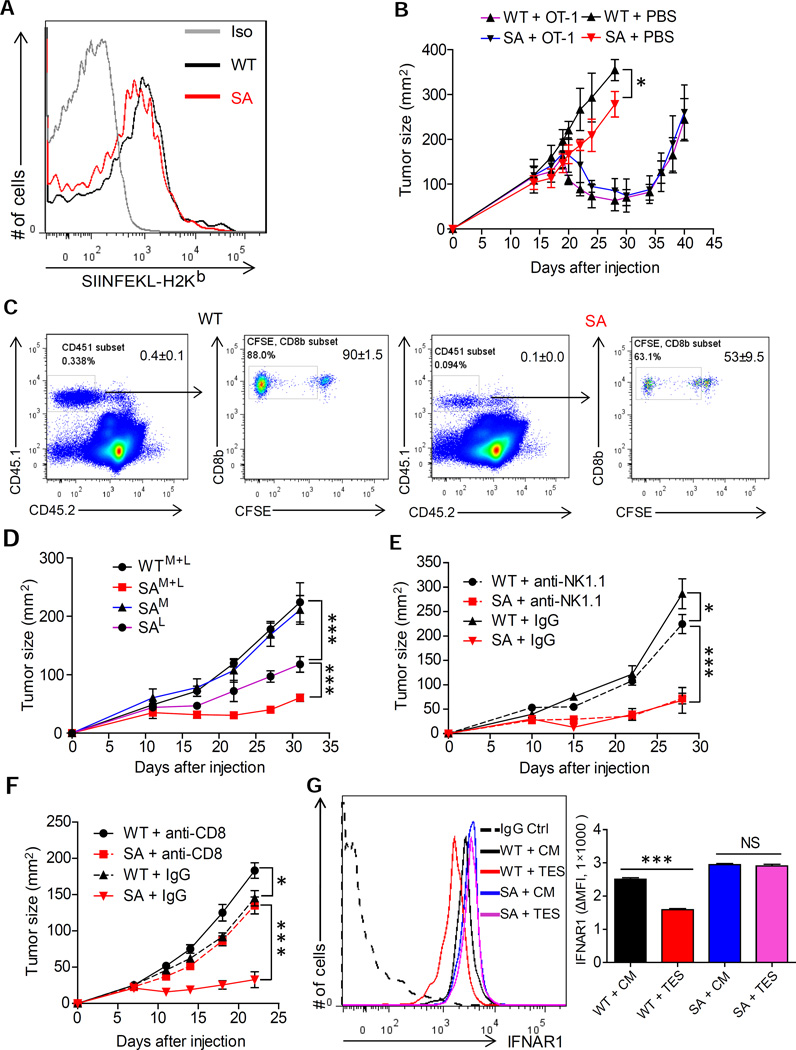
We next transplanted MC38 tumor cells ectopically expressing ovalbumin (MC38OVA) into immune-deficient Rag1−/− mice that harbored either WT or SA IFNAR1. Dendritic cells (CD45+ MHC-II+CD103+ CD11c+) isolated from either WT or SA tumors presented a comparable levels of MHC-I-complexed OVA peptide (Figure 4A). Consistent with these data, adoptive transfer of WT OVA-specific OT-1 CTL into these Rag1−/− mice resulted in an initial decrease in tumor volume (followed by subsequent re-growth of tumors) regardless of IFNAR1 status (Figure 4B).
Figure 4. Downregulation of IFNAR1 on cytotoxic T lymphocytes promotes tumor growth.
(A) Representative FACS analysis of levels of MHC-I-complexed OVA peptide on the surface of intra-tumoral CD45+MHC-II+CD103+CD11c+ DCs isolated from the MC38OVA tumors grown in Rag1−/− mice harboring WT or SA IFNAR1.
(B) Growth of MC38-OVA tumors in Rag1−/− mice harboring WT or SA IFNAR1 after adoptive transfer of WT-OT-1 T cells injected on Day 18 after tumor inoculation.
(C) Representative flow cytometry analysis of the percentage (left panels) and proliferation (right panels) of CD8+CD45.1+ at day 7 in the spleens from WT and SA mice after adoptive transfer of naive CFSE-labeled OT-1 T cells at day 0 and subsequent challenge with MC38OVA cells at day 1.
(D) Growth of MC38 tumors in Rag1−/− mice that received bone marrow containing WT or SA myeloid and lymphoid cells (WTM+L and SAM+L) or WT myeloid and SA lymphoid cells (SAL) or WT lymphoid and SA myeloid cells (SAM).
(E) MC38 tumor growth in WT or SA mice treated with anti-NK.1.1 or IgG control antibodies.
(F) MC38 tumor growth in WT or SA mice treated with anti-CD8 or IgG control antibodies.
(G) Representative FACS analysis and quantification (n=4, each in triplicates) of IFNAR1 levels on the surface of CD3+ CD8+ cells isolated from WT or SA spleens and incubated in vitro with control media (CM) or tumor explant supernatant (TES) for 2 hr.
Data depicted as Mean±SEM (n=4–6); similar results were obtained in at least 2 independent experiments.
See also Figure S4.
We next injected WT or SA mice with naive OT-1 T cells followed by challenge with MC38OVA and subsequent assessment of numbers and proliferation of splenic OT-1 CD8+ T cells six days later. Under these conditions, even a lesser CTL proliferation was seen in the SA hosts compared to WT mice (Figure 4C) further indicating that the tumor growth defect observed in SA mice is unlikely to depend on increased antigen presentation by SA DCs.
We next generated mixed bone marrow chimeras in which myeloid and/or lymphoid cells from either WT or SA animals were used to reconstitute bone marrow in lethally irradiated WT mice (Diamond et al., 2011). These chimeric mice harbored comparable numbers of myeloid and lymphoid cells and expected IFNAR1 levels on these cells in the spleen (Figure S4C). A dramatic suppression of MC38 tumor growth observed in chimeras that received both lymphoid and myeloid cells from SA donors (“SAM+L”, Figure 4D and S4D) was indicative of critical importance of IFNAR1 downregulation in the bone marrow-derived cells. Relative to this group, only a slight acceleration of tumorigenesis was seen in mice that received WT myeloid cells and SA lymphocytes (“SAL”). This result indicate that, while there is a role for IFNAR1 expressed on myeloid cells, maintaining the levels of IFNAR1 on lymphocytes appears to be critical for the tumor growth suppression.
Indeed, while depletion of NK cells in SA mice did not alter growth of MC38 tumors (Figure 4E and S4E), depletion of CD8+ cells notably stimulated tumor growth in SA animals (Figure 4F and S4F). We next sought to determine whether IFNAR1 can be downregulated specifically on the intra-tumoral CD8+ T cells. Incubation of WT (but not SA) CD8+ T cells with the tumor explant supernatant (Figure 4G) or MC38 cell conditioned media (Figure S4G) notably downregulated IFNAR1 cell surface levels. Together with notably lower levels of IFNAR1 on the surface of CD3+CD8+ WT (but not SA) cells isolated from tumors compared to those isolated from spleens (Figure S4H), these data suggest that tumor conditions trigger downregulation of IFNAR1 on the surface of CTL and that this downregulation contributes to aggressive tumorigenesis.
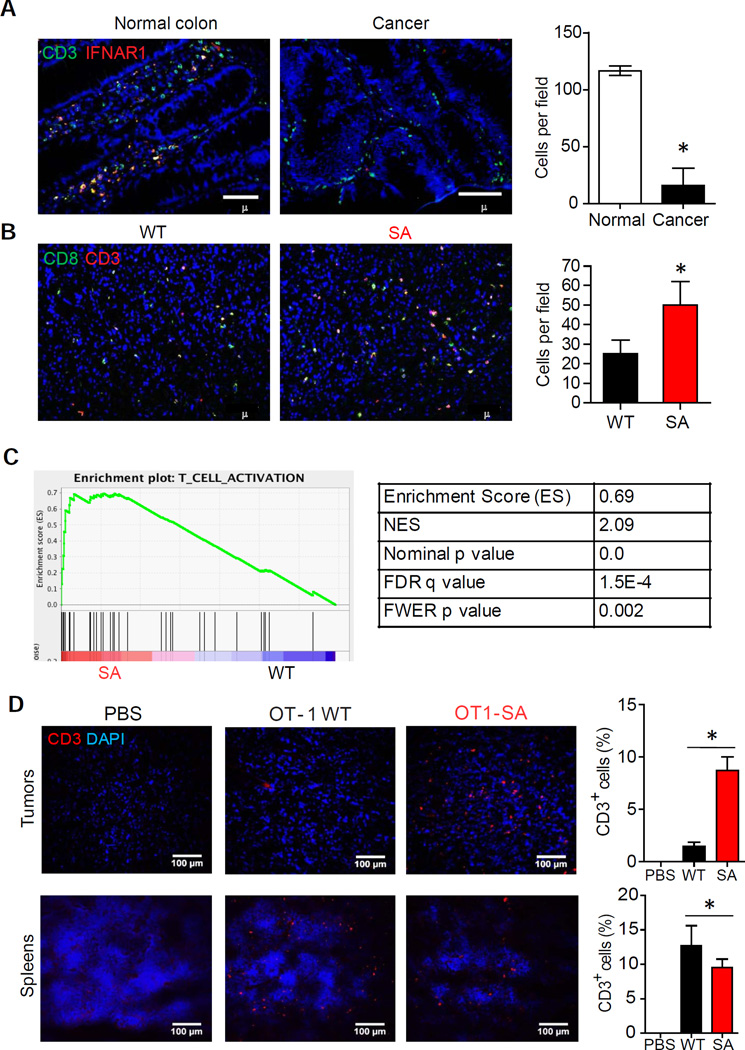
A majority of cells expressing high levels of IFNAR1 in normal human colon were CD3+ cells (Figure S5A). Importantly, in human CRC tissues, most IFNAR1-positive T lymphocytes were peripheral to the tumor and very few of them were found inside human tumors (Figure 5A and S5B). Whereas these low numbers of CTL found in human CRC could be recapitulated in MC38 tumors grown in WT mice, a greater absolute and relative number of the CD3+CD8+ CTL were found within tumors developed in SA animals (Figure 3F and 5B). Consistent with this result, mouse SA tumors exhibited a prominent T cell gene expression signature (Figure 5C) and particularly high levels of mRNA of genes indicative of T cell effector function including Ifng and Gzmb (Figure S5C) as well as increased levels of IFNγ and granzyme B proteins in MC38 tumor lysates (Figure S5D). Likewise, greater levels of Ifng mRNA expression were also observed in SA tumors induced by AOM-DSS treatment compared to WT tumors (Figure S1G). These results suggest that reduced accumulation of CTL (indicative of immune privileged niche) in CRC is associated with IFNAR1 downregulation.
Figure 5. Downregulation of IFNAR1 in cytotoxic T lymphocytes contributes to development of the immunosuppressive tumor microenvironment in CRC.
(A) Representative immunofluorescent analysis and quantification of IFNAR1hiCD3+ T cells infiltration of human normal colon and CRC. Tissue sections were stained with antibodies against IFNAR1 (red) and CD3 (green) and contrasted with DAPI (blue). At least 20 randomly chosen fields from each of 8 patient samples for each group were quantified.
(B) Representative immunofluorescent analysis and quantification of CD3+CD8+ T cells infiltration of MC38 tumors from WT and SA mice. Tumor sections were stained with anti CD3 (red) and CD8 (green) antibodies and contrasted with DAPI (blue). At least 20 randomly chosen fields from each of 5 tumor samples were quantified.
(C) GSEA results of the T-cell activation gene signature in WT and SA tumors.
(D) Representative immunofluorescent analysis of T cells found in spleens or MC38OVA tumors grown in Rag1−/− mice after treating these mice with PBS or adoptive transfer of WT or SA OT-1 T cells (2×107 per mouse). Data depicted as % of CD3+ cells among all DAPI-stained cells and are representative of at least 20 random fields scored from tissues of 4 mice.
Data are shown as Mean+SEM.
See also Figure S5.
Accordingly, studies involving adoptive transfer of CD8+ T cells into Rag1−/− mice burdened with MC38OVA tumors revealed a greater intra-tumoral accumulation of CTL derived from SA-OT-1 mice compared to CTL from WT-OT-1 animals (Figure 5D). These results suggest that the status of IFNAR1 on CTL determines their ability to accumulate within tumors. These findings in human and mouse tumors collectively indicate that IFNAR1 downregulation on CTL that occurs within CRC tumors prevents CTL accumulation, thereby establishing a local immune privileged tumor microenvironment.
Given that little, if any, difference was detected in proliferation of SA and WT CD8+ T lymphocytes activated in vitro (Figure S5E) or isolated from tumor bearing mice (Figure S5F), we next focused on other mechanisms that could explain preferential CTL accumulation in tumors of SA mice. Cancer-associated fibroblasts positive for the fibroblast activation protein (FAP) produce CXCL12 chemokine that prevent intra-tumoral CTL buildup in a mouse pancreatic cancer model (Feig et al., 2013). Intriguingly, activated SA and WT CTL exhibited a similar chemotaxis towards CXCL12 or CXCL9 (Figure S5G), suggesting that retaining IFN signaling may not necessarily increase the migratory abilities of CTL.
Downregulation of IFNAR1 on cytotoxic T lymphocytes undermines their survival within tumor microenvironment
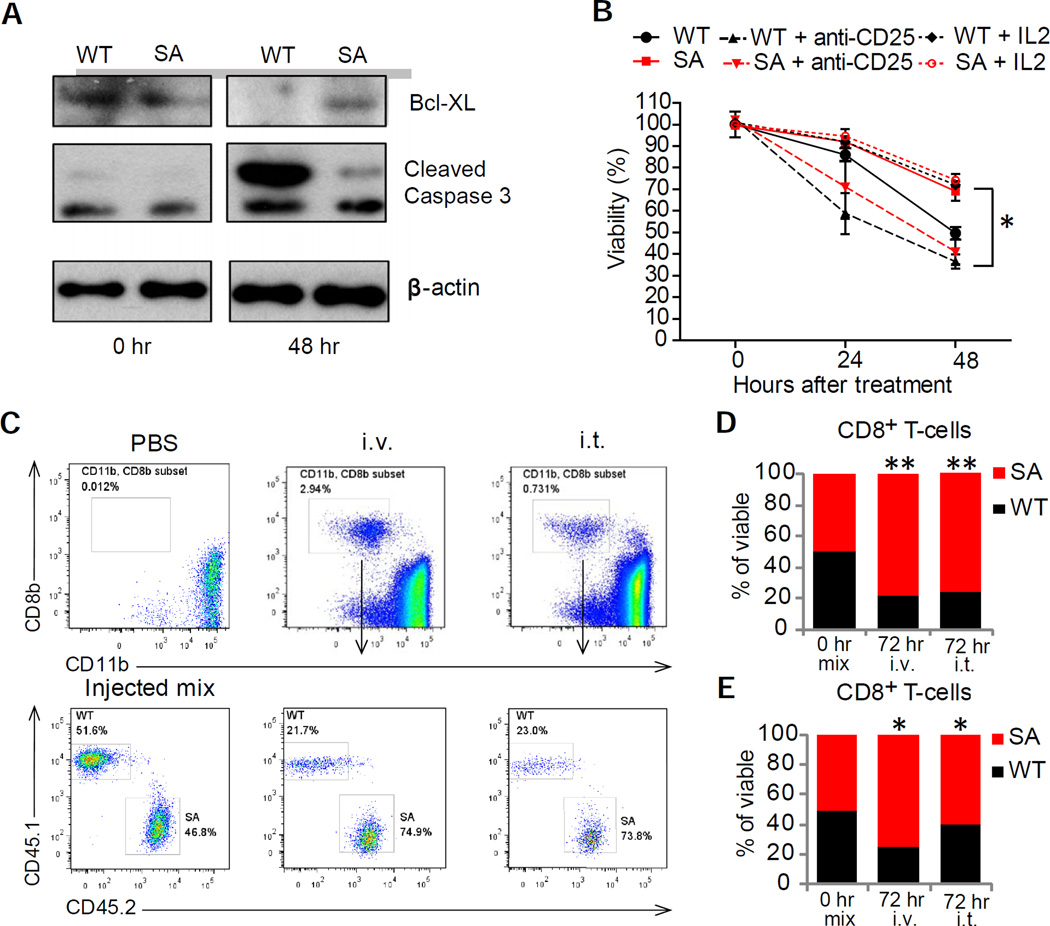
IFN promote survival of anti-viral CTL by protecting them from killing by NK cells (Crouse et al., 2014; Xu et al., 2014). Depletion of NK cells in Rag1−/− mice indeed increased the total number of transferred T cells but did not affect a greater viability of SA T cells compared to WT T cells (Figure S6). Given this result and observation that NK depletion did not alter tumorigenesis in either WT or SA mice (Figure 4E), we focused on other mechanisms by which downregulation of IFNAR1 may affect anti-tumor CTL. Antigen-exposed SA CTL cultured in vitro maintained greater levels of IFNAR1 and the mRNA and the protein levels for the anti-apoptotic regulator Bcl-XL and lower levels of cleaved caspase-3 compared with WT CTL (Figure 6A and S7A). Accordingly, a decrease in cell viability was more pronounced in WT cells than in SA cells under these conditions (Figure 6B). Importantly, while pre-treatment with interleukin-2 (IL2) increased viability of WT CTL, neutralizing the IL2 receptor using anti-CD25 antibody undermined the survival of SA CTL (Figure 6B). We further found that SA CTLs produced notably more IL2 (Figure S7B) and expressed greater levels of IL2Rα mRNA and protein compared to WT cells (Figure S7C–D). Thus, it is likely that downregulation of IFNAR1 promotes death of activated CTL by attenuating the pro-survival effects of the IL2 pathway.
Figure 6. Downregulation of IFNAR1 on cytotoxic T lymphocytes undermines their survival within tumor microenvironment.
(A) Immunoblot analysis of Bcl-xl, cleaved caspase 3 levels and β-actin (loading control) in splenocytes from WT and SA OT-1 mice activated with SIINFEKL peptide (0.5µg/mL for 48 hr) and then cultured for indicated times.
(B) Viability of activated CD3+CD8+ cells in the presence of media supplemented or not with either IL2 (100 U/ml) or anti-CD25 antibody (100 µg/ml) as indicated was determined by flow cytometry analysis after indicated times. Mean ±SD (triplicates per each mouse spleen – average from 3 mice) are shown. Asterisks denote statistical significance (p<0.05) between WT and SA, between WT and WT treated with IL2 and between SA and SA treated with anti-CD25 antibody.
(C) Flow cytometry analysis of the fraction of viable OT-1 WT (CD45.1) or SA (CD45.2) CTL in the MC38OVA tumors 72 hr after injection (1:1 ratio) intravenously (i.v.) or directly into the tumors (i.t.) of Rag1−/− mice bearing MC38OVA tumors.
(D) Quantitation of the experiments shown in 6C (Mean % of viable cells from tumors from 3–5 mice). Similar results were obtained at least in 2 independent experiments.
(E) Quantitation of flow cytometry analysis of the fraction of viable FAP-CAR EGFP+ WT (CD45.1) or EGFP+ SA (CD45.2) CTL in the MC38 tumors 72 hr after injection (1:1 ratio) intravenously (i.v.) or directly into the tumors (i.t.) of WT MC38 tumor bearing mice. Data are shown as Mean % of viable cells (n=5).
See also Figures S6 and S7.
Activated SA OT-1 CTL exhibited greater viability than WT OT-1 CTL when these cells were simultaneously injected at 1:1 ratio intravenously or directly into the MC38OVA tumors grown in Rag1−/− mice (Figure 6C–D). This result suggests that stabilization of IFNAR1 on antigen-specific CTLs improves their survival within the tumors. To further corroborate this possibility, we used the chimeric antigen receptor (CAR)-based approach that involved the introduction of the CAR against FAP (FAP-CAR, (Wang et al., 2014)) into WT or SA CTL. We generated FAP-CAR T cells separately from WT or SA lymphocytes, and then mixed these cells in equal parts prior to adoptive transfer (as a 1:1 mixture) into MC38 tumor-bearing WT mice (Figure S7E). Regardless of the route of administration (intra-tumoral or intravenous), a greater fraction of SA cells was found in the tumor 3 days later (Figure 6E and S7F). These results suggest that TME-induced downregulation of IFNAR1 on CTLs compromises viability of these CTLs inside tumors.
Downregulation of IFNAR1 in CTL limits the efficacy of anti-cancer therapies
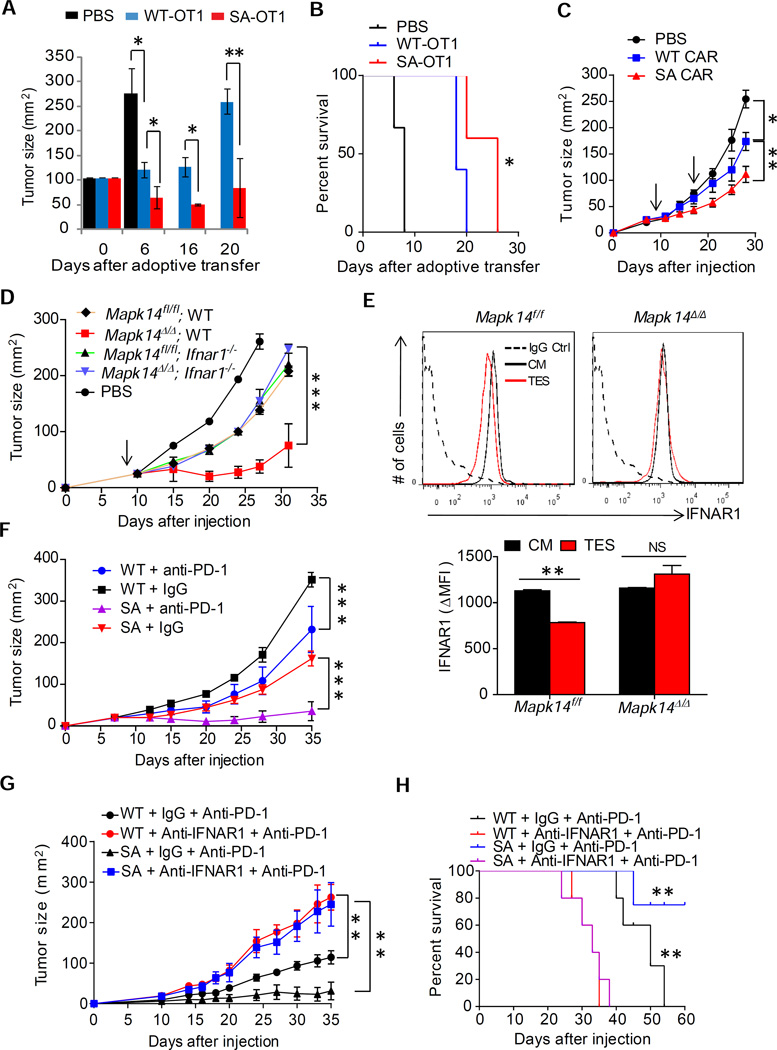
We next examined the importance of IFNAR1 downregulation in modulating the efficacy of adoptive CTL transfer-based immunotherapy. Adoptive transfer of WT OT-1 lymphocytes into Rag1−/− mice bearing MC38OVA tumors was much less efficient in sustained suppression of tumor growth and prolonging animal survival compared to SA OT-1 cells (Figure 7A–B). Furthermore, FAP-CAR CTLs prepared from SA cells exhibited a substantially greater therapeutic effect against MC38 tumors relative to FAP-CAR WT CTL (Figure 7C). The magnitude of these effects is probably underappreciated because the SA allele may inhibit proliferation of CAR CTLs (data not shown) and FAP-CAR SA cells used in these experiments were likely to partially suppress IFN signaling downstream of the receptor.
Figure 7. Downregulation of IFNAR1 in CTL limits the efficacy of immunotherapies.
(A) Anti-tumor effects of adoptively transferred OT-1-SA and OT-1 WT T cells in MC38OVA tumor-bearing Rag1−/− described in Figure 5D.
(B) Kaplan-Meier analysis of survival of MC38 tumor-bearing mice from panel A.
(C) Anti-tumor effects of IFNAR1 WT and IFNAR1 SA FAP-CAR T cells (time of administration indicated by arrow) in MC38 tumor-bearing mice
(D) Growth of MC38 tumors in WT mice that received PBS or FAP-CAR T cells harboring indicated status of Mapk14 and Ifnar1 shown.
(E) Cell surface IFNAR1 levels on CD3+CD8+splenocytes of indicated genotype treated with tumor explant supernatant (TES) or control media (CM) for 2 hr. Representative FACS and quantitation (below) are shown.
(F) Anti-tumor effect of anti-PD-1 antibody administration in WT mice and SA mice bearing MC38.
(G) Effect of IFNAR1 neutralization on the efficacy of anti-PD-1 treatment of WT or SA mice bearing MC38 tumors
(H) Kaplan-Meier analysis of survival of MC38 tumor-bearing mice from panel G. Data shown as Mean±SEM (n=3–5) from each of at least 2–3 independent experiments.
To overcome this problem we sought to acutely stabilize IFNAR1 via inducible ablation of p38α - a kinase potentially involved in the ligand-independent downregulation of IFNAR1 (Bhattacharya et al., 2011). We prepared FAP-CAR T cells from the splenocytes of mice harboring floxed Mapk14 (gene that encodes p38α), either Ifnar1+/+ (WT) or Ifnar1−/− alleles and either no Cre or inducible Ubc9-CreERT2. These FAP-CAR T cells were treated with 4-hydroxytamoxifen and then injected into WT mice burdened with MC38 tumors. As seen from Figure 7D, inactivation of p38α in IFNAR1-expressing FAP-CAR CTL (Mapk14Δ/Δ WT) dramatically increased the anti-tumor efficacy of these cells. Importantly, this increased effect could be negated by concurrent ablation of Ifnar1 suggesting that most (if not all) effects of p38α deletion depend on sustained IFNAR1 signaling within CTL (Figure 7D). Together with inability of CTL lacking p38α to downregulate IFNAR1 in response to an in vitro treatment with tumor explant supernatant (Figure 7E), these data provide genetic evidence suggesting that tumor-derived factors-induced p38α-dependent downregulation of IFNAR1 on the surface of CTLs limits the efficacy of CAR-based therapeutics in solid tumors.
Intriguingly, a fraction of MC38 tumors that did not get rejected in SA mice eventually reached a larger size (A1, Figure 3A). Whereas SA tissues retained a greater immune response and robust IFN signatures, there was also a notable increase in expression of PD-L1/CD274 checkpoint molecule (Figure S3C). Accordingly, treatment with anti-PD-1 antibody at the dose that only slightly delayed MC38 tumors growth in WT mice caused a robust therapeutic effect leading to a stable disease in SA mice (Figure 7F). Importantly, anti-PD-1 therapy was notably less efficient in suppressing tumor growth (Figure 7G) and improving animal survival (Figure 7H) in SA mice that also received anti-IFNAR1 neutralizing antibody. These data collectively suggest that downregulation of IFNAR1 undermines the efficacy of checkpoint-targeted immunotherapeutics against solid tumors.
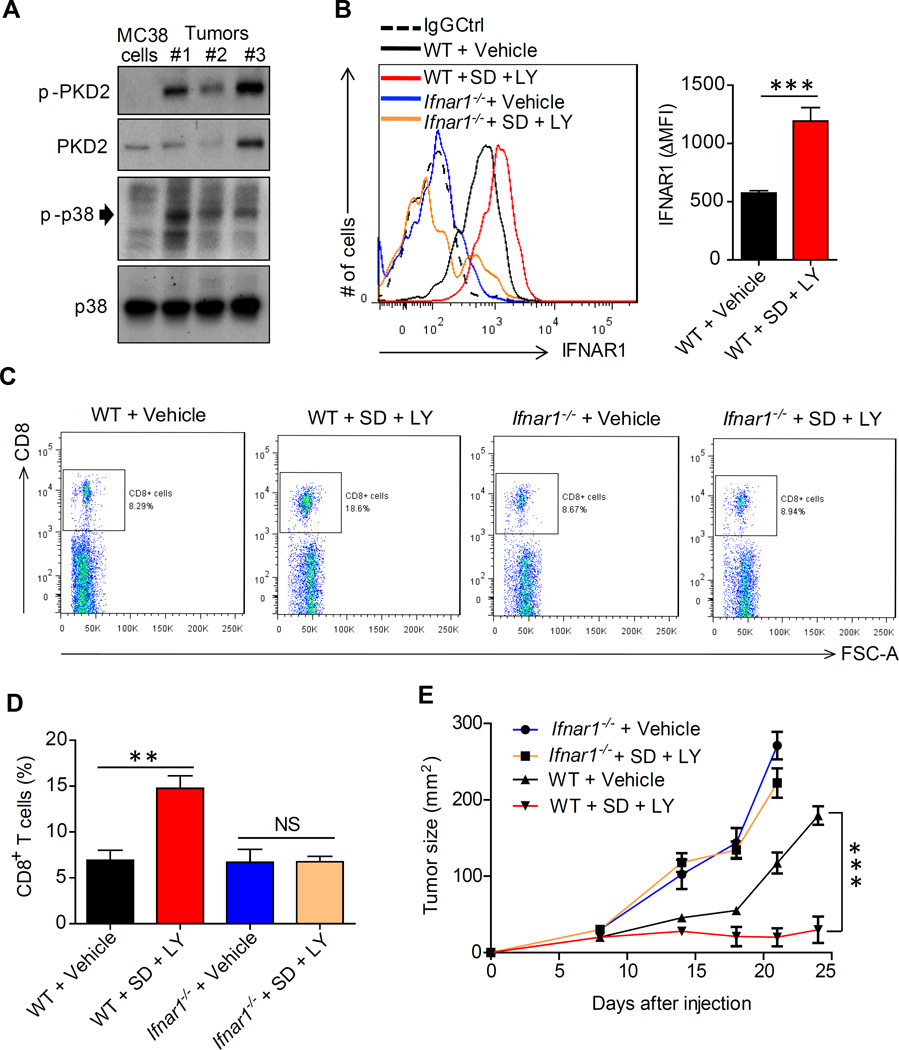
We noted a greater phosphorylation of p38α in lysates from MC38 tumors relative to cultured MC38 cells (Figure 8A), consistent with activation of p38α by TME stress. Accordingly, we next examined whether downregulation of IFNAR1 can be reversed by pharmacologic means. To this end, we attempted to stabilize IFNAR1 using the p38 inhibitor LY2228820 (LY). Given that some TME factors such as vascular endothelial growth factor can also downregulate IFNAR1 via activating protein kinase-2 (PKD2) (Zheng et al., 2011), and that PKD2 activity was indeed increased in MC38 tumors compared to cultured MC38 cells (Figure 8A) we combined the p38 inhibitor with SD-208 (SD), a PKD inhibitor.
Figure 8. Pharmacologic stabilization of IFNAR1 disrupts immune privileged niche and elicits a therapeutic effect against tumors.
(A) Immunoblot analysis of phosphorylation and levels of p38α and PKD2 kinases in cultured MC38 cells and MC38 tumors.
(B) Levels of cell surface IFNAR1 on tumor infiltrating CD3+CD8+ cells isolated from MC38 tumors grown in WT or Ifnar1-null mice treated with kinase inhibitors as indicated. Representative FACS and quantification are shown.
(C) Frequency of CD8+ T cells (% of CD45+ cells) isolated from MC38 tumors grown in WT or Ifnar1-null mice treated with kinase inhibitors as indicated.
(D) Quantification of results shown in panel C.
(E) Anti-tumor effect of SD-208 and LY2228820 administered to Ifnar1−/− and WT mice bearing MC38 tumors as described in Methods.
Data are shown as Mean±SEM (n=5 from each of 3 independent experiments).
See also Figure S8
The combination of these small molecules (LY+SD) prevented tumor explant supernatantinduced downregulation of IFNAR1 on CTL in vitro (Figure S8A). Furthermore, in vivo treatment with this combination (which was well tolerated by tumor-bearing mice, Figure S8B), led to a notable increase in the overall levels of IFNAR1 within tumors (Figure S8C) and specifically of cell surface IFNAR1 levels on intra-tumoral CD3+CD8+ cells (Figure 8B). Remarkably, administration of LY+SD robustly increased numbers of CTLs found inside MC38 tumors that grew in WT but not in Ifnar1−/− mice (Figures 8C–D and S8D–E) suggesting that inhibition of p38 and PKD disrupts the immune privileged niche within the TME in an IFNAR1-dependent manner.
Consistent with an important role of IFNAR1 downregulation in stimulation of tumorigenesis, treatment with these kinase inhibitors dramatically suppressed growth of MC38 tumors in WT mice (Figure 8E) but not in mice lacking Ifnar1 (Figure 8E) indicating that stimulation of IFNAR1 signaling is a major mechanism underlying the immune-reactivating and anti-tumorigenic effects of these agents. In all, these results provide a proof of principle for pharmacologic stabilization of IFNAR1 as the means to attenuate local immunosuppression within tumors and to suppress tumor growth.
Discussion
Delineating the mechanisms that impose localized immune suppression within the TME is essential for improving the efficacy of immunotherapeutics in solid tumors. Here we present evidence that links the TME stress-driven downregulation of IFNAR1 to reduced viability of intra-tumoral CTL and ensuing establishment of immune-privileged niche in CRC tumors. A decrease in IFNAR1 levels and expression of IFN-inducible genes found in human CRC tumors and recapitulated in mouse tumors is associated with the establishment of a localized niche virtually void of CTL, as well as with robust tumor growth and poor prognosis. Downregulation of IFNAR1 specifically in CTL induced by tumor-associated factors inhibits CTL viability and undermines the efficacy of immune therapies. Conversely, genetic or pharmacologic stabilization of IFNAR1 disrupts the immune privileged niche, suppressed tumor growth and increases the efficacy of CAR T cell therapy and immune checkpoint inhibitors.
These findings suggest that IFNAR1 downregulation contributes to development and progression of CRC. While not arguing against additional role of IFNAR1 in IFN-modulated regulation of antigen presentation and activation of dendritic cells (Diamond et al., 2011; Fuertes et al., 2011; Hildner et al., 2008), stromal resistance to tumor-induced angiogenesis (Zheng et al., 2011), and other processes, our current results strongly indicate that IFNAR1 downregulation on intra-tumoral CTL contributes to the establishment of immune-privileged TME by undermining CTL survival. These results are consistent with an important role of IFN as an activation signal for T cells (Curtsinger et al., 2005) and observation that insufficient “third signal” contributes to the inhibition of CTL in solid tumors (Curtsinger et al., 2007). It appears that downregulation of IFNAR1 on CTL negatively affects responses of these CTL to IL2 pro-survival signals and, accordingly, stimulates pro-apoptotic pathways, although other mechanisms cannot be ruled out. Regardless of the exact mechanisms, data presented here argue for development of the therapeutic strategies aimed to stabilize IFNAR1 and improve CTL viability within solid tumors.
Our results specifically emphasize the importance of IFNAR1 downregulation on CTL. Given importance of these cells in anti-tumor immunity against diverse malignant lesions, it is likely that downregulation of IFNAR1 in the stromal compartment may stimulate growth and progression of other cancer types. Indeed, we have recently demonstrated that loss of IFNAR1 stimulates growth of transplanted melanomas (Katlinskaya et al., 2016). Overall, our data are consistent with intra-tumoral IFN production being linked with CTL generation and viability (Hiroishi et al., 2000), observations that IFN may act to improve the effect of adoptive transfer of CTL (Hervas-Stubbs et al., 2012) and with recent finding that a specific CAR design, which serendipitously increased IFN signaling in CTL, evoked an augmented therapeutic effect (Zhao et al., 2015). Nevertheless, our results do not rule out additional putative cellular targets (e.g. interleukin-10-expressing Treg cells (Stewart et al., 2013) and additional mechanisms by which elimination of IFNAR1 and suppression of IFN signaling can further contribute to localized immunosuppression and stimulation of solid tumors growth.
Previous studies utilizing chemically induced and transplantable sarcomas and melanomas in IFNAR1 knockouts have identified specific CD8α+ DCs as targets of protective role of IFN against tumors (Diamond et al., 2011; Fuertes et al., 2011; Hildner et al., 2008). Functional defects of Ifnar1-null DCs reported in these studies are consistent with an important role of IFNAR1 in maturation of DCs (Le Bon and Tough, 2002; Santini et al., 2009). We did not observe an increase in antigen presentation in SA mice. Furthermore, SA DCs might have a survival disadvantage given that elimination of IFNAR1 plays an important role in preserving the viability of IFN-expressing DCs exposed to inducers of pathogen recognition receptor signaling (Qian et al., 2011). Future use of SA animals in sarcoma and melanoma models is likely to reveal additional information on the relative contribution of IFNAR1 status in DCs and other leukocytes to anti-tumor immunity.
Genetic and pharmacologic studies described here provide a proof of principle for a focus on stabilization of IFNAR1 to increase the efficacy of immunotherapies against CRC and possibly other solid tumors. Whereas the mechanisms underlying the therapeutic effect of p38/PKD inhibitors are likely to be mediated by many cell types (in addition to CTL), it is noteworthy that these inhibitors still act in an IFNAR1-dependent manner. In addition to targeting p38 and PKD kinases responsible for phosphorylation of IFNAR1 leading to recruitment of the SCF-βTrcp E3 ligase, it might be possible to inhibit this class of ligases. Cullin-dependent ligases (including SCF-βTrcp) can be targeted by inhibiting NEDD9-activating enzyme; its selective inhibitor MLN4924 is currently under clinical trials in solid tumors (Sarantopoulos et al., 2015). Additional studies on combining IFNAR1 stabilizing regimens with diverse immunotherapeutic approaches are currently in progress.
EXPERIMENTAL PROCEDURES
A detailed description of the procedures utilized in this work can be found in Supplemental Experimental Procedures. Use of pre-existing previously collected under informed consent human archival de-codified and de-identified CRC tissue arrays and samples that could not be directly or indirectly linked to individual human subjects was exempt from the institutional review. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania and were carried out in accordance with the IACUC guidelines. All mice were on the C57Bl/6 background and had water ad libitum and were fed regular chow. Mice were maintained in a specific pathogen-free facility in accordance with American Association for Laboratory Animal Science guidelines. Littermate animals from different cages were randomly assigned into the experimental groups. These randomized experimental cohorts were either co-housed or systematically exposed to other groups’ bedding to ensure equal exposure to all group’s microbiota. Statistical analysis was performed using Microsoft or GraphPad Prism 7 software. Unpaired Student t test was used for the comparison between two groups. One-way ANOVA or two-way ANOVA analysis followed by the Bonferroni post-hoc test were used for the multiple comparisons. Repeated-measure two-way ANOVA (mixed-model) followed by the Bonferroni post-hoc test was used for the analysis of tumor growth curve. A value of p <0.05 was considered significant.
Data from the global expression profiling studies were collected with Illumina BeadStudio 3.1.1.0 software, and statistical analyses were conducted on the IlluminaGUI R-package. Gene sets from microarray data were analyzed for overlap with curated data sets (C5, H) in MSigDB using the web interface available at http://www.broadinstitute.org/gsea/msigdb/index.jsp. The raw data have been deposited to NCBI (accession number GSE76889).
For AOM/DSS colorectal carcinogenesis, co-housed experimental mice were intraperitoneally injected with 10 mg/kg azoxymethane (Sigma). A week later, they were supplied with tap water containing 2.5% dextran sodium sulfate (TdB Consultancy) for 7 days, followed by 14 days of regular water. This cycle was repeated three times and mice were sacrificed 2 weeks after the end of the last DSS cycle or at the end of 10 weeks. Colons were harvested, washed of feces with DPBS, and slit open longitudinally to count tumors. Tumors were flash frozen in liquid nitrogen or embedded into OCT media for subsequent analysis.
Supplementary Material
SIGNIFICANCE.
Understanding the mechanisms by which solid tumors suppress anti-tumor immunity is critical for success of immune therapies. Here we demonstrate that tumor microenvironment factors-induced downregulation of type I interferon receptor IFNAR1 is a central mechanism underlying the ability of tumor microenvironment to undermine viability of cytotoxic T cells and to generate intra-tumoral immune privileged niches devoid of these cells. Means preventing the loss of IFNAR1 eliminate these niches, inhibit tumor growth and increase the efficacy of immunotherapies utilizing checkpoint inhibitors or chimeric antigen receptor T cells. These findings delineate a mechanism of localized intra-tumoral immune suppression and prompt the development of IFNAR1-stabilizing agents for their use in anti-cancer immune therapies.
Highlights.
Colorectal tumors downregulate interferon receptor IFNAR1
Loss of IFNAR1 promotes generation of immune privileged niche
IFNAR1 regulates viability of cytotoxic lymphocytes and efficacy of immunotherapies
Pharmacologic stabilization of IFNAR1 suppresses tumor growth
Acknowledgments
This work was supported by the NIH/NCI PO1 CA165997 grant (to J.A.D, C.K. and S.Y.F.), and RO1 CA092900 (to S.Y.F. and H.R.). Additional support from T32 CA009140 (to K.V.K.) is also appreciated. We thank Ze’ev Ronai, Mark J. Smyth, Melissa Wong, Yibin Wang, Susan Weiss and Susan Ostrand-Rosenberg for reagents, Sandra Pellegrini, Mathias Muller, Birgit Strobl, and the members of Gabrilovich, Fuchs, Diehl, Koumenis, Pear and Minn labs for critical suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION: S.Y.F., K.V.K., J.G., E.P., H.R., C.K., and J.A.D. designed the research; K.V.K., J.G., A.O., Y.V.K., R.C., C.J.C., S.B., D.P.B., M.G., A.R.P., P.C. and A.K.R. performed the experiments and interpreted the data; S.Y.F., K.V.K., J.G., E.P., H.R., C.K., and J.A.D. wrote the manuscript with the help of all authors.
All authors declare no competing financial interests.
REFERENCES
- Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, HuangFu WC, Dong G, Qian J, Baker DP, Karar J, Koumenis C, Diehl JA, Fuchs SY. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene. 2013;32:4214–4221. doi: 10.1038/onc.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Katlinski KV, Reichert M, Takano S, Brice A, Zhao B, Yu Q, Zheng H, Carbone CJ, Katlinskaya YV, et al. Triggering ubiquitination of IFNAR1 protects tissues from inflammatory injury. EMBO Mol Med. 2014;6:384–397. doi: 10.1002/emmm.201303236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Qian J, Tzimas C, Baker DP, Koumenis C, Diehl JA, Fuchs SY. Role of p38 protein kinase in the ligand-independent ubiquitination and downregulation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2011;286:22069–22076. doi: 10.1074/jbc.M111.238766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012;18:1224–1231. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2:187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J Interferon Cytokine Res. 2013;33:211–225. doi: 10.1089/jir.2012.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18:377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Hellstrom I, Hellstrom KE, Pierce GE, Yang JP. Cellular and humoral immunity to different types of human neoplasms. Nature. 1968;220:1352–1354. doi: 10.1038/2201352a0. [DOI] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Mancheno U, Riezu-Boj JI, Larraga A, Ochoa MC, Alignani D, Alfaro C, Morales-Kastresana A, Gonzalez I, Larrea E, et al. CD8 T cell priming in the presence of IFN-alpha renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J Immunol. 2012;189:3299–3310. doi: 10.4049/jimmunol.1102495. [DOI] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Riezu-Boj JI, Gonzalez I, Mancheno U, Dubrot J, Azpilicueta A, Gabari I, Palazon A, Aranguren A, Ruiz J, et al. Effects of IFN-alpha as a signal-3 cytokine on human naive and antigen-experienced CD8(+) T cells. Eur J Immunol. 2010;40:3389–3402. doi: 10.1002/eji.201040664. [DOI] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroishi K, Tuting T, Lotze MT. IFN-alpha-expressing tumor cells enhance generation and promote survival of tumor-specific CTLs. J Immunol. 2000;164:567–572. doi: 10.4049/jimmunol.164.2.567. [DOI] [PubMed] [Google Scholar]
- Huangfu WC, Qian J, Liu C, Liu J, Lokshin AE, Baker DP, Rui H, Fuchs SY. Inflammatory signaling compromises cell responses to interferon alpha. Oncogene. 2012;31:161–172. doi: 10.1038/onc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- Katlinskaya YV, Katlinski KV, Yu Q, Ortiz A, Beiting DP, Brice A, Davar D, Sanders C, Kirkwood JM, Rui H, et al. Suppression of Type I Interferon Signaling Overcomes Oncogene-Induced Senescence and Mediates Melanoma Development and Progression. Cell Rep. 2016;15:171–180. doi: 10.1016/j.celrep.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22:5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Liu J, HuangFu WC, Kumar KG, Qian J, Casey JP, Hamanaka RB, Grigoriadou C, Aldabe R, Diehl JA, Fuchs SY. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009;5:72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta D, Karar J, Jenkins WT, Kumanova M, Jenkins KW, Tobias JW, Baldwin D, Hatzigeorgiou A, Alexiou P, Evans SM, et al. In vivo profiling of hypoxic gene expression in gliomas using the hypoxia marker EF5 and laser-capture microdissection. Cancer Res. 2011;71:779–789. doi: 10.1158/0008-5472.CAN-10-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S, Yoshida H, Moodley D, LeBoite H, Rothamel K, Raj T, Ye CJ, Chevrier N, Zhang SY, Feng T, et al. Parsing the Interferon Transcriptional Network and Its Disease Associations. Cell. 2016;164:564–578. doi: 10.1016/j.cell.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Qian J, Zheng H, Huangfu WC, Liu J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Pathogen recognition receptor signaling accelerates phosphorylation-dependent degradation of IFNAR1. PLoS Pathog. 2011;7:e1002065. doi: 10.1371/journal.ppat.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr C, Kerick M, Fischer A, Kuhn A, Kashofer K, Timmermann B, Daskalaki A, Meinel T, Drichel D, Borno ST, et al. High-throughput miRNA and mRNA sequencing of paired colorectal normal, tumor and metastasis tissues and bioinformatic modeling of miRNA-1 therapeutic applications. PLoS One. 2013;8:e67461. doi: 10.1371/journal.pone.0067461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, Chapman R, Hertzog PJ. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini SM, Lapenta C, Santodonato L, D'Agostino G, Belardelli F, Ferrantini M. IFN-alpha in the generation of dendritic cells for cancer immunotherapy. Handb Exp Pharmacol. 2009:295–317. doi: 10.1007/978-3-540-71029-5_14. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos J, Shapiro GI, Cohen RB, Clark JW, Kauh JS, Weiss GJ, Cleary JM, Mahalingam D, Pickard MD, Faessel HM, et al. Phase I Study of the Investigational NEDD8-Activating Enzyme Inhibitor Pevonedistat (TAK-924/MLN4924) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-1338. [DOI] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, Leighty RM, Roers A, Karp CL, Muller W, Trinchieri G. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123:4859–4874. doi: 10.1172/JCI65180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Kohrer K, Rahbar R, Diefenbach A, et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Condomines M, van der Stegen SJ, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis. Blood. 2011;118:4003–4006. doi: 10.1182/blood-2011-06-359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS, Elpek K, et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.