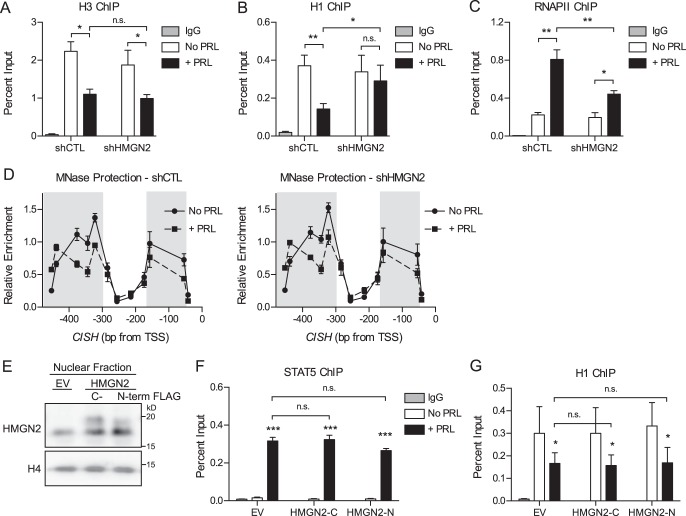
FIGURE 6.
HMGN2 facilitates the loss of histone H1 but does not affect nucleosome core particle remodeling. A–C, HMGN2 knockdown impairs H1 dissociation and RNAPII loading at CISH but does not affect the dissociation of H3. T47D cells expressing shCTL or shHMGN2 were treated with or without PRL for 45 min and were analyzed by ChIP-qPCR at the CISH promoter. Nuclear lysates were precipitated with antibodies against H3 (A), H1 (B), or RNAPII (C). Normal IgG served as a nonspecific control. CISH primers are described in the legends to Figs. 2 and 5A. The amount of DNA recovered was calculated relative to the input control and is graphed as a percentage of input. Results are presented as the mean ± S.E. (error bars), n ≥ 3 independent experiments. Statistical significance was determined by two-way repeated measures ANOVA. D, HMGN2 knockdown does not affect nucleosome core particle positioning by MNase accessibility. T47D cells expressing shCTL or shHMGN2 were treated with or without PRL for 1 h. Nuclei were permeabilized, and the chromatin was digested with MNase. Mononucleosomal DNA was purified, and MNase protection was determined by qPCR using amplicons tiling across the CISH promoter (Fig. 3A). Enrichment of mononucleosomal DNA was calculated relative to amplification of undigested genomic DNA. Values are plotted at the midpoint of each amplicon. Results are presented as the mean ± S.E. of three independent experiments. Statistical significance was determined by two-way repeated measures ANOVA. The shaded region indicates statistical significance between the PRL-treated and untreated conditions, p ≤ 0.05. The shCTL and shHMGN2 conditions were not significantly different. E, HMGN2 overexpression in T47D cells. Shown is Western blotting analysis of cells stably infected to express exogenous HMGN2 with either a C-terminal (HMGN2-C) or N-terminal FLAG tag (HMGN2-N) or an empty vector (EV) control. Nuclear lysates were probed with an antibody against HMGN2, and histone H4 was used as a loading control. FLAG-tagged HMGN2 runs slightly higher than endogenous HMGN2. F and G, HMGN2 overexpression does not further promote STAT5 binding or H1 dissociation at CISH. Cells from E were analyzed by ChIP-qPCR as in A–C, using antibodies against STAT5 (F) or H1 (G). Results are presented as the mean ± S.E. of three independent experiments. Statistical significance was determined by two-way repeated measures ANOVA. n.s., p > 0.05; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.