FIGURE 4.
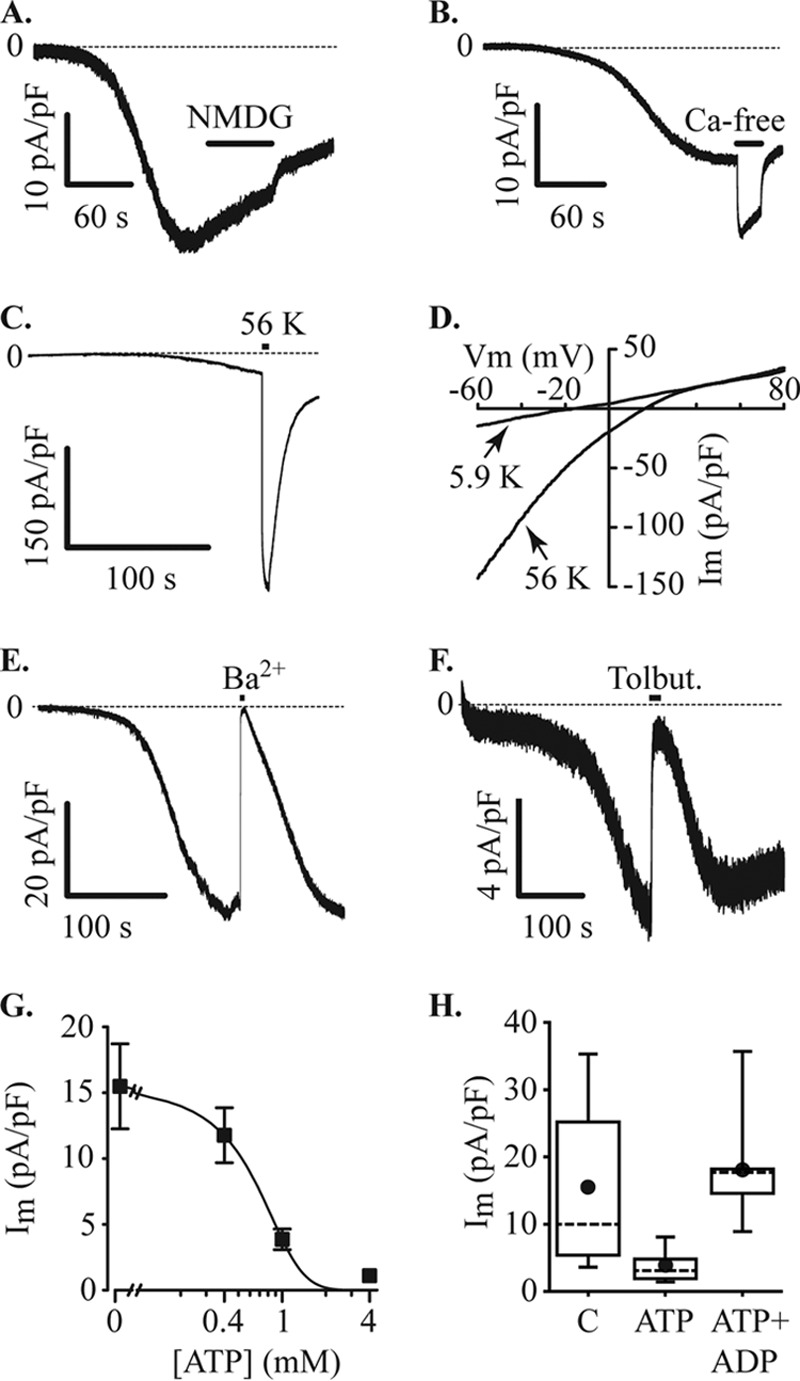
Inward currents through KATP channels. MIN6 cells were voltage-clamped at −60 mV in the whole cell configuration with a Cs+ pipette solution containing 200 μm EGTA. Under these recording conditions, with normal bath solution, an inward current developed, and the effects of ion substitution were tested. A, the current was not inhibited by substituting extracellular Na+ with NMDG+ (NMG, application indicated by filled bar). B, the inward current amplitude increased upon removal of extracellular Ca2+ (Ca-free, filled bar). C, raising extracellular K+ from 5.9 to 56 mm (56 K, filled bar) produced an increase in current amplitude (−30.9 ± 9.1 pA/pF at normal extracellular K+ and −247.2 ± 75.0 pA/pF at 56 mm K+, n = 7). D, increasing K+ produced a positive shift of the reversal potential for the current. In the cell illustrated, the reversal potential shifted from −16.1 mV to +15.9 mV and recovered to −14.3 mV, the mean change was from −17.6 ± 2.5 mV at normal (5.9 mm K+) to +13.0 ± 3.1 mV at 56 mm K+ and −15.2 ± 2.2 after returning to normal K+. Im, inward current. E, substitution of extracellular Ca2+ for Ba2+ also inhibited the inward current. Current inhibition caused by a 10-s pulse of Ba2+ was 97.1 ± 1.2% (n = 7). F, the inward current was reversibly inhibited by 100 μm tolbutamide (Tolbut). G, increasing concentrations of ATP in the pipette solution inhibited the inward current (Im). Data points were curve-fitted indicating an IC50 of 0.6 mm (data from 9–13 cells at each concentration). H, inhibition of inward current (Im) by 1 mm ATP was reversed by 1 mm ADP (C, control (no ATP), n = 13 cells; ATP, n = 9 cells; ATP+ADP, n = 9 cells).
