FIGURE 4.
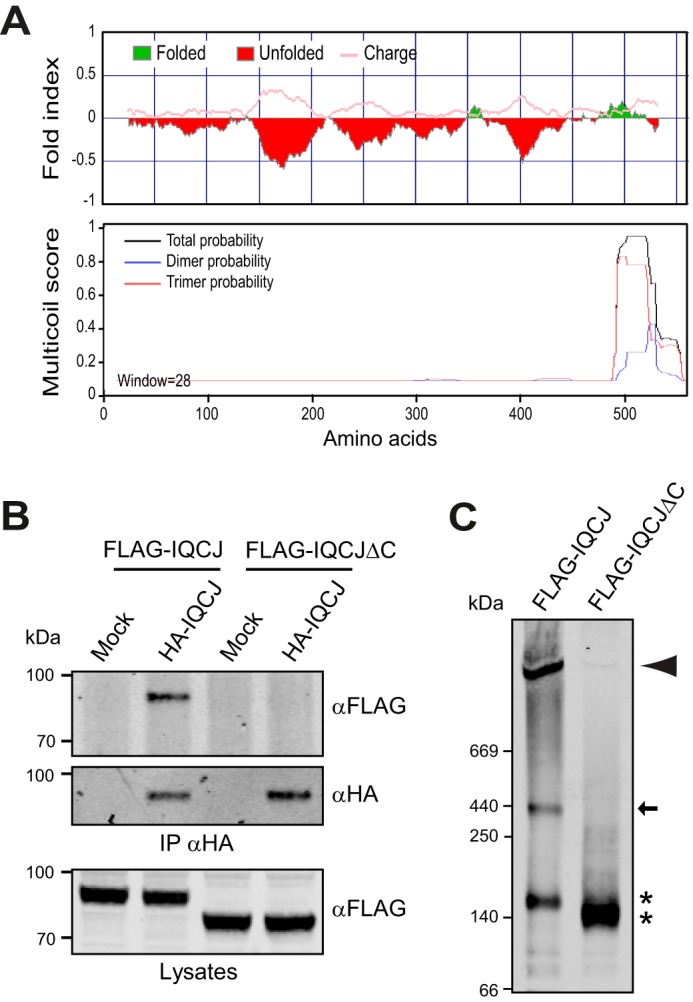
IQCJ-SCHIP1 self-associates through its C-terminal domain. A, probabilities of unstructured and charged regions (upper panel) and oligomerization (lower panel) along the mouse IQCJ-SCHIP1 amino acid sequence predicted with the FoldIndex and MULTICOILS software. B, IP on lysates from transfected COS-7 cells overexpressing HA-tagged IQCJ-SCHIP1 (HA-IQCJ) and FLAG-IQCJ or FLAG-IQCJΔC with HA antibodies and revealed by immunoblotting with FLAG and HA antibodies. Crude protein extracts (Lysates) were immunoblotted to verify protein expression. FLAG-IQCJ co-immunoprecipitates with HA-IQCJ, whereas FLAG-IQCJΔC does not. C, lysates from cells expressing FLAG-IQCJ or FLAG-IQCJΔC were resolved on native polyacrylamide gels and analyzed by immunoblotting with FLAG antibodies. Three proteins bands are detected for FLAG-IQCJ, whereas only one band is detectable for FLAG-IQCJΔC. * indicates the positions of FLAG-IQCJ and FLAG-IQCJΔC monomers (∼140 kDa). The arrow indicates the band that may correspond to trimers (∼440 kDa), and the arrowhead the band that may correspond to higher order oligomers (>1000 kDa). Mock, transfection with an empty vector. Molecular mass markers are shown in kDa on the left of the panels.
