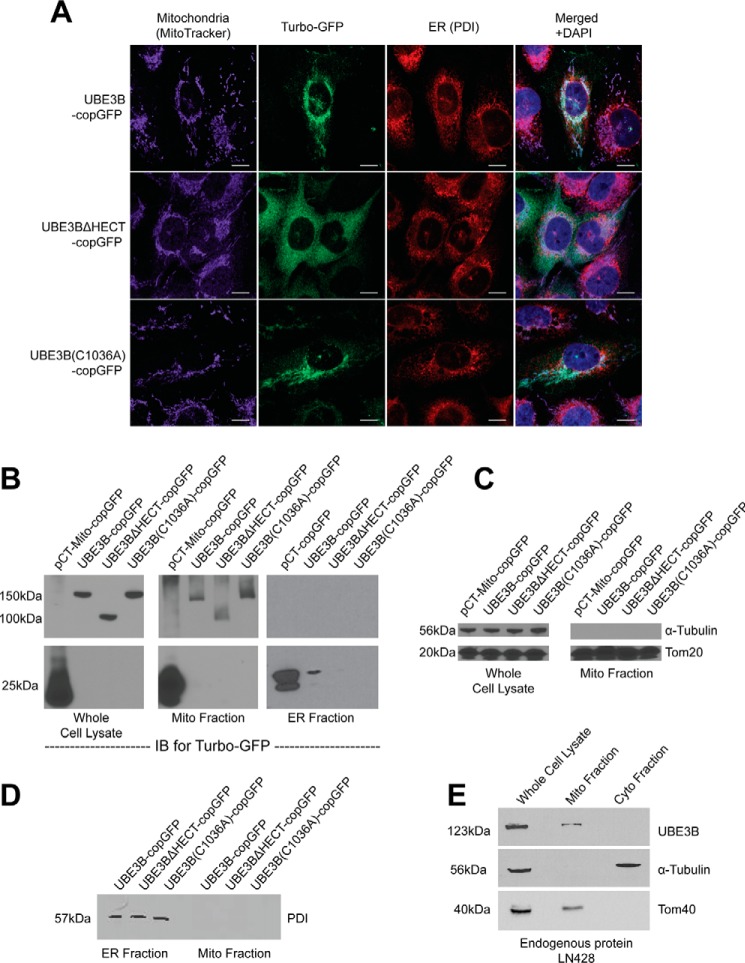
FIGURE 2.
UBE3B is associated with mitochondria. A, LN428 cells were transduced with lentivirus to stably express UBE3B, UBE3BΔHECT, or UBE3B(C1036A), all with C-terminal copGFP tags, and then were fixed and imaged with a Nikon A1rsi confocal microscope. MitoTracker DeepRed (excitation wavelength, 647 nm; emission wavelength, 665 nm) was used to stain mitochondria before fixation; cells were then immunostained for PDI, a marker for the endoplasmic reticulum (excitation wavelength, 568 nm; emission wavelength, 602 nm). DAPI (excitation wavelength, 360 nm; emission wavelength, 460 nm) was used to counterstain nuclei, as seen in the merged images. B, to confirm the immunofluorescence results, subcellular fractionation of the stable cell lines was performed, resulting in isolation of mitochondrial, ER, and cytoplasmic fractions, which were then probed by immunoblot (IB). An antibody against TurboGFP was used to detect UBE3B-copGFP proteins. Full-length UBE3B-copGFP proteins were detected only in mitochondrial fractions and in whole cell lysate. To confirm the quality of fractionation, fractions were probed with markers for different cellular compartments. C, mitochondrial fractions lack the cytoplasmic marker α-tubulin and show enrichment of the mitochondrial marker Tom20. D, purity of the ER fraction was assessed by immunoblot probe for the ER marker PDI, showing no cross-contamination with the mitochondrial fraction. E, to show that endogenous UBE3B associates with mitochondria and the immunofluorescence and subcellular fractionation results in A–D are not artifacts of overexpression or of the copGFP tag, we performed subcellular fractionation and immunoblot analysis for endogenous UBE3B in LN428 cells, using the cytoplasmic marker α-tubulin and the mitochondrial marker Tom40 to confirm fractionation.