FIGURE 4.
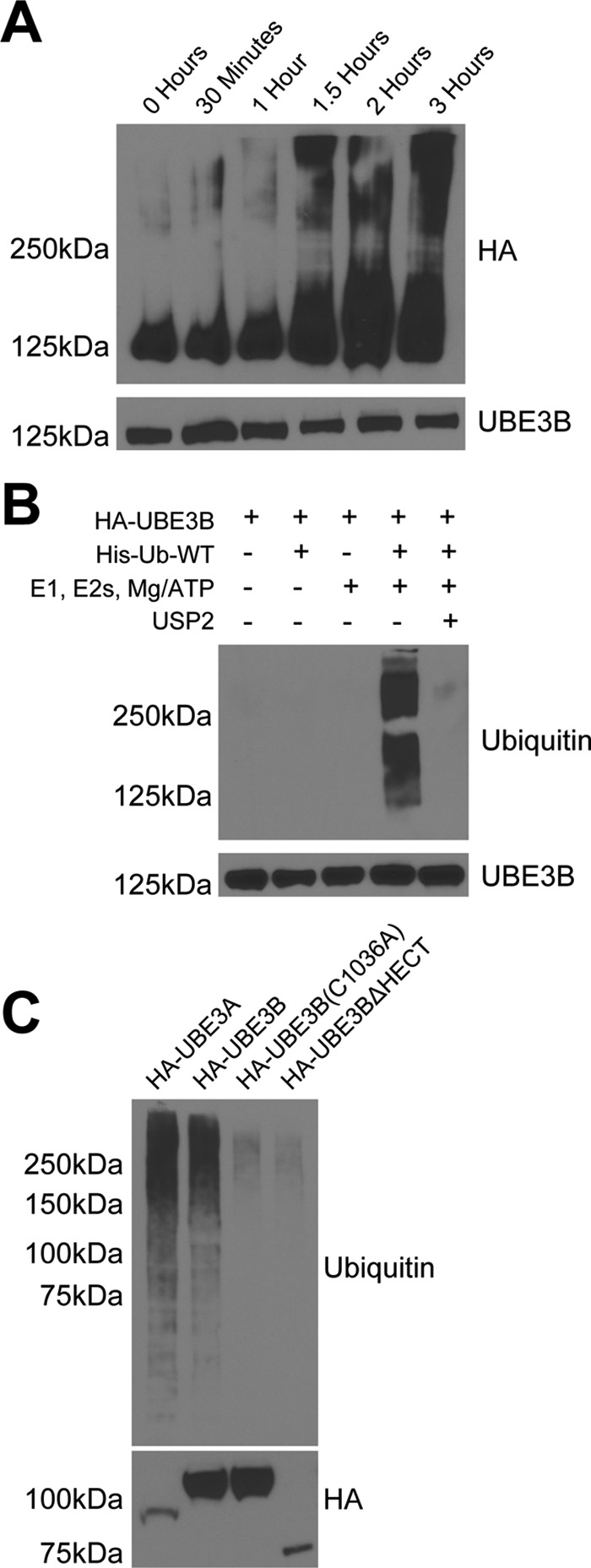
UBE3B exhibits ubiquitin ligase activity. Cell lysates from LN428 cells that stably express UBE3A, UBE3B, UBE3BΔHECT, or UBE3B(C1036A), all with N terminus HA tags, were immunoprecipitated using anti-HA affinity matrix and subjected to ubiquitylation activity assays. The ubiquitylation assays were carried out in 30-μl reactions with 0.1 μm E1, 0.25 μm of an E2 mixture, 1 μm ubiquitin aldehyde, 0.75 μg/μl His-ubiquitin, and 1× magnesium/ATP mixture, unless otherwise noted. A, ubiquitylation activity assay was performed in vitro using His-tagged wild type ubiquitin (His-Ub-WT). The expected size of HA-UBE3B is 125 kDa. Note the time-dependent increase in the high molecular mass signal. B, polyubiquitin chain formation was lost when ubiquitin or E1, E2s, or ATP were removed from the reaction mixture, or if 20 μg of the catalytic core of the deubiquitinating enzyme USP2 was added to a completed reaction for 30 min at 37 °C (63). C, polyubiquitin chains were lacking in reactions containing the catalytically inactive (HA-UBE3B(C1036A)) or HECT deleted (HA-UBE3BΔHECT) mutants of UBE3B. Completed reactions were applied to immunoblot for analysis of ubiquitylation with anti-hemagglutinin (HA) or ubiquitin (Ub) antibodies. Bottom panel shows comparable loading of the immunoprecipitated proteins. Representative immunoblots from three independent experiments are shown. Antibodies used for each of the immunoblots are listed on the side of the panels.
