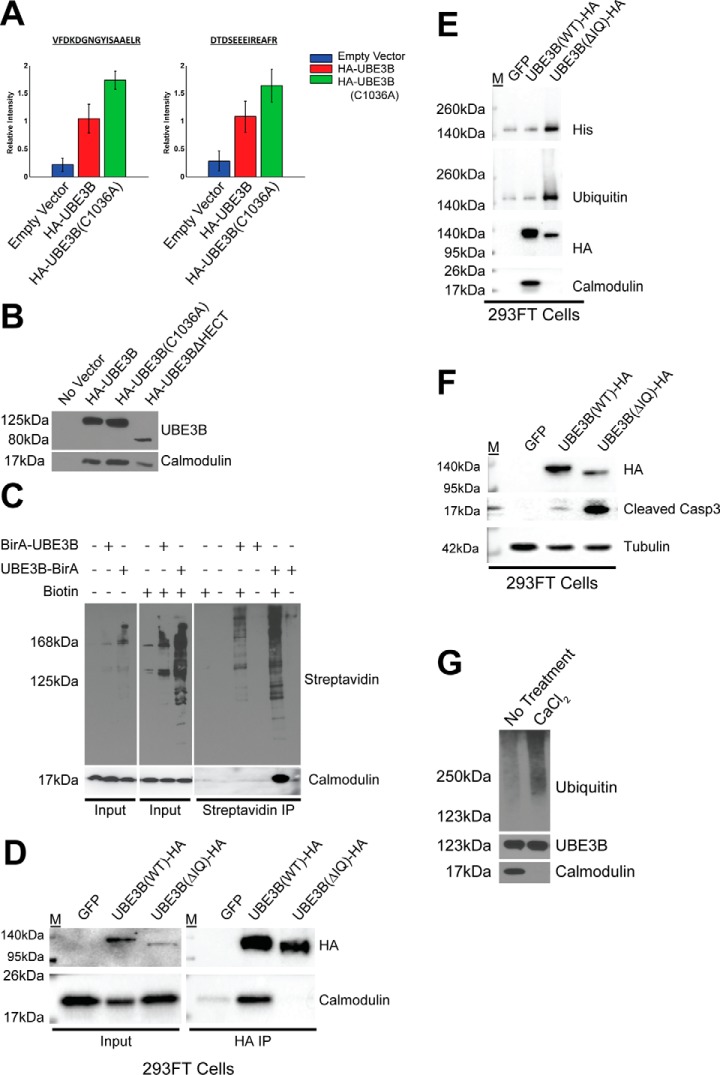
FIGURE 5.
UBE3B associates with calmodulin through the IQ motif in a calcium-dependent manner. A, quantification results of HA-Ab affinity purification and high resolution LC-MS analysis for two proteotypic calmodulin peptides, VFDKDGNGYISAAELR and DTDSEEEIREAFR. Bar graphs represent the standardized mean values ± S.E. following affinity purification and differential mass spectrometry analysis of LN428 cells containing an empty vector control or expressing wild type HA-tagged UBE3B (HA-UBE3B) or the catalytic inactive UBE3B with an HA tag (HA-UBE3B(C1036A)). B, cells, indicated in the figure, were lysed, and the HA-tagged proteins were immunoprecipitated, as described under “Experimental Procedures,” to identify the proteins that interact with UBE3B, UBE3B(C1036A), or UBE3BΔHECT. Shown is an immunoblot to confirm the interaction of calmodulin with UBE3B as seen by LC-MS using anti-UBE3B and calmodulin antibodies. C, BioID analysis was performed using BirA-UBE3B and UBE3B-BirA with streptavidin immunoprecipitated to confirm that HA-UBE3B and calmodulin interact. Cells were treated with biotin (5 μm) for 24 h before harvest, as indicated. Streptavidin-purified proteins were then probed by immunoblot using streptavidin-HRP. Bottom right panel indicates the identification of calmodulin after streptavidin capture. D, 293FT cells were transiently transfected (48 h) with a plasmid expressing WT UBE3B-HA or UBE3BΔIQ-HA followed by immunoprecipitation with HA-affinity matrix beads. Deletion of the IQ motif demolished the interaction between UBE3B and calmodulin. E, 239FT cells were transiently transfected (48 h) with a plasmid expressing WT UBE3B-HA or UBE3BΔIQ-HA followed by immunoprecipitation with HA-affinity matrix beads. A ubiquitylation assay was then performed as described under “Experimental Procedures.” The results were analyzed by immunoblot using anti-ubiquitin, anti-His, and anti-HA antibodies to detect UBE3B and anti-CaM antibody to detect calmodulin. Ubiquitylation is increased in UBE3BΔIQ due to the loss of loss of the interaction with calmodulin. F, 239FT cells were transiently transfected (48 h) with a plasmid expressing WT UBE3B-HA or UBE3BΔIQ-HA. The expression of UBE3BΔIQ-HA triggered cell apoptosis as indicated by an elevation of the cleaved form of caspase3. G, calmodulin interaction with UBE3B is lost when immunoprecipitation beads are washed with CaCl2, and ubiquitylation is increased when calmodulin is not bound to UBE3B. Immunoprecipitations and the bead-based in vitro assay were performed as described above.