FIGURE 8.
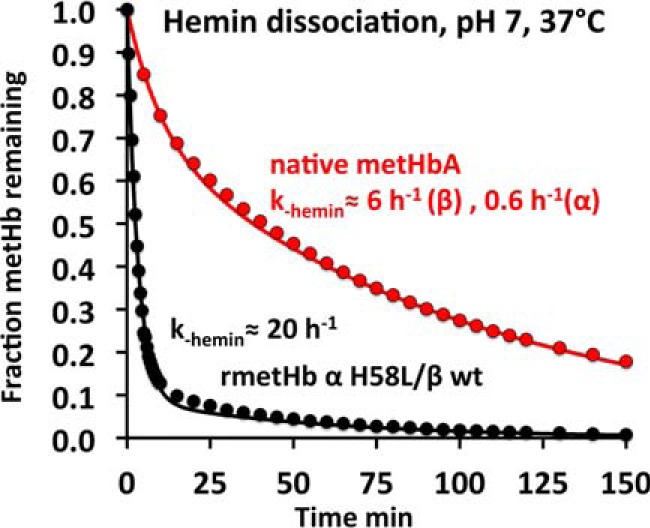
Time courses for hemin dissociation from native metHbA and met-rHb Kirklareli in 0. 1 m phosphate buffer at pH 7, 37 °C. The “brown” metHb sample at 10 μm was mixed with 100 μm H64Y/V68F apoMb, which turns “green” when it scavenges hemin, and with a 10-fold excess of the scavenging agent, the observed rates represent the rate of hemin dissociation from the Hb sample (23, 72). As shown, the rate of hemin dissociation from the mutant α subunits in rHb Kirklareli is roughly 30 times faster than from WT α subunits.
