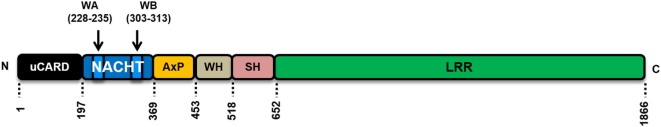
Figure 1.
Schematic representation of the human NLRC5 protein structure and domain organization. uCARD, untypical caspase activation and recruitment domain (CARD); this domain shows only low sequence similarities to canonical CARD domains. A nuclear localization signal is located in this region. NACHT domain, involved in the nucleotide bindings and presumable important for oligomerization and activation of NLRC5. WA and WB are Walker A and Walker B motifs, respectively. The WA motif is responsible for nucleotide triphosphate binding; WB motif is responsible for nucleotide triphosphate hydrolysis. WH, winged helix domain. SH, superhelical domain, contains eight α-helices, function unknown. LRR, leucine-rich repeat, protein–protein interaction domain, responsible for ligand binding in other pattern-recognition receptors.