FIGURE 5.
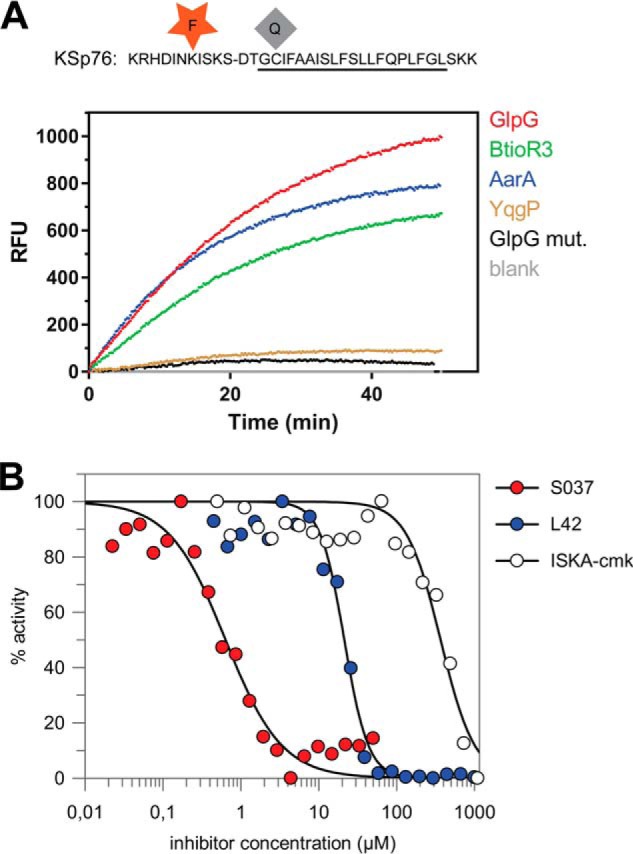
Red-shifted variant of the LacYTM2-based fluorogenic substrate. A, modification of Lys in the P5 position of KSp31 by the red-shifted TAMRA fluorophore and P4′ Cys by a dark quencher QXL610 yields highly fluorogenic substrate KSp76 that is efficiently cleaved by rhomboid proteases GlpG, AarA, YqgP, and BtioR3 at identical concentrations to those used in Fig. 3G. Excitation wavelength was 553 nm, and emission was followed at 583 nm. B, the red-shifted fluorogenic substrate KSp76 allows measurement of inhibition by compounds that absorb in the UV region, such as isocoumarin, and is thus suitable for high-throughput screening. The dose-response curves of the chloromethylketone ISKAcmk, β-lactam L42, and isocoumarin S037 were measured after a 60-min preincubation of enzyme with inhibitor. The curves were fitted in GraFit 7 to yield apparent IC50 values.
