FIGURE 5.
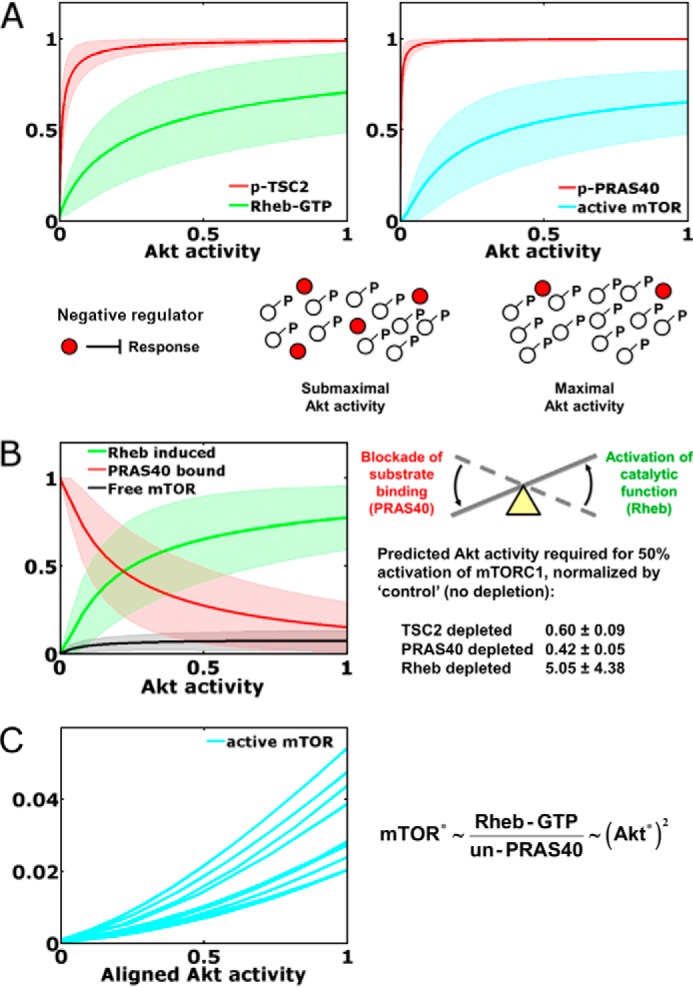
Dynamical motifs encoded in Akt/mTORC1 signaling dynamics. A, neutralization of negative regulators. Ensemble predictions of the model at steady state (mean ± S.D., n = 10,000) show that phosphorylation (p) of the negative regulators TSC2 and PRAS40 approach saturation at low stoichiometries of Akt activation. This offers maximal sensitivity of Rheb-GTP loading and mTORC1 activation, because TSC2 and PRAS40 regulate those responses in their unphosphorylated states. B, seesaw regulation of mTORC1. Steady-state analysis of the model predicts the status of the mTORC1 complex (mean ± S.D., n = 10,000), with a transition from largely PRAS-inhibited and largely Rheb-induced states. Also shown are the model-predicted Akt activity levels, normalized by that of the control, required to elicit half-maximal activation of mTORC1 in cells with TSC2, PRAS40, or Rheb depleted by a nominal 80% (mean ± S.D., n = 10,000). C, as shown for 10 randomly selected parameter sets, seesaw regulation of mTORC1 encodes an ultrasensitive, nonlinear input-output relationship over the relevant range of Akt activity (i.e. with Akt activity scaled as it was in the alignment to phospho-Akt data, where a value of 1 corresponds to the mean of the 1 nm PDGF time course). Asterisks indicate kinase activity. UN-PRAS40, unphosphorylated PRAS40.
