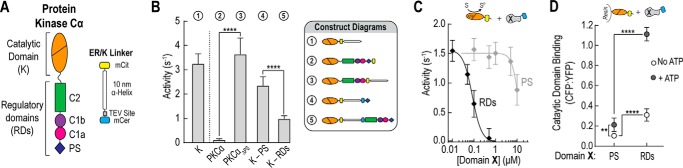
FIGURE 1.
Pseudosubstrate is not sufficient for autoinhibition of PKCα. A, schematic of PKCα and the 10-nm ER/K linker cassette used in FRET sensors. Fluorophores used in the majority of experiments are mCerulean (mCer, FRET donor) and mCitrine (mCit, FRET acceptor). B, effector-independent activity of the catalytic domain of PKCα (K) compared with full-length PKCα with and without the PS domain and for catalytic domain sensors containing either the PS (K-PS) alone or all regulatory domains (K-RDs). ATPase activity resulting from ATP consumption during the phosphorylation reaction was monitored in the presence of 50 μm MBP peptide and 50 μm ATP at 21–22 °C and reported as mole of ATP per mol of protein per s or s−1. C, activity of the catalytic domain of PKCα in the presence of increasing PS domain or intact RDs. ATPase activity measurements were taken with 10 μm of both MBP peptide and ATP and corrected for background activity. D, ensemble binding of either PS alone or the RDs region to resin-bound catalytic domain in the presence or absence of 1 mm ATP. Binding was quantified by monitoring mCerulean (PS or RDs; 475 nm) and mCitrine (catalytic domain; 525 nm) fluorescence. To account for nonspecific interactions, binding to unlabeled resin was background subtracted. For all experiments, data are derived from at least three independent protein preparations with at least two measurements for each condition per preparation (mean ± S.E., n ≥ 3). In the schematics in C and D, Domain X represents either the PS or the RDs region of PKCα.