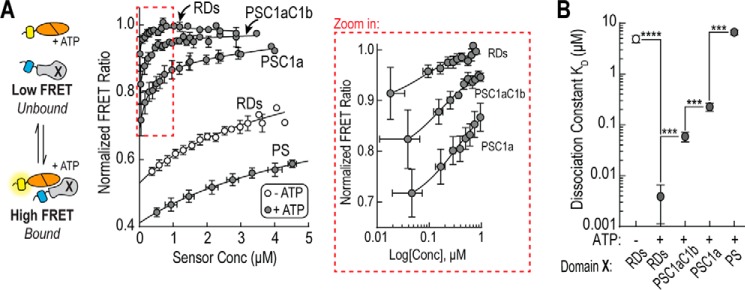
FIGURE 4.
Regulatory domains increase affinity of PS-catalytic domain interaction. A, equilibrium binding using bimolecular FRET. Right, bimolecular FRET ratio (mCit/mCer) as a function of protein concentration in the presence (dark circles) or absence (empty circles) of 1 mm ATP. Left, the region highlighted by the red box is re-plotted using a logarithmic scale. In the schematic, Domain X represents the region of the regulatory domain being used for binding experiments. Sensors were digested with TEV protease to cleave sensors into mCit-tagged catalytic domain and mCer-tagged regulatory domains. The bimolecular interaction was fit (solid line) as described under “Experimental Procedures” (18). B, average equilibrium constant (KD) for regulatory domain regions in the presence (dark circles) or absence (empty circle) of 1 mm ATP. Dissociation constants are summarized in Table 2. Data are derived at least three independent protein preparations with at least two measurements for each condition per preparation (mean ± S.E., n ≥ 3). ***, p ≤ 0.001; ****, p ≤ 0.0001 (Student's unpaired t test).