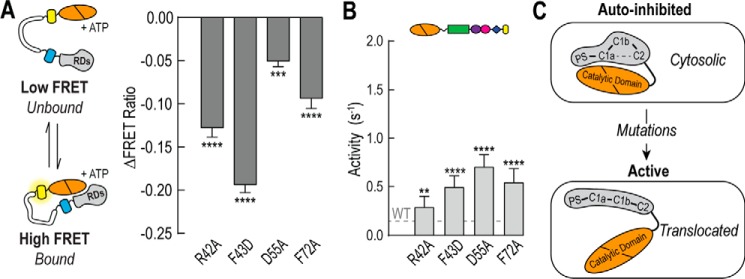
FIGURE 6.
C1a mutations disrupt PKCα autoinhibition. A, the effect of C1a mutations on regulatory domain binding to the catalytic domain by FRET in the presence of 100 μm ATP. B, effector-independent ATPase activity of full-length PKCα containing C1a point mutations. For all FRET and activity measurements, data are derived from at least three independent protein preparations with at least two measurements for each condition per preparation (mean ± S.E., n ≥ 3). C, the C1a domain coordinates intramolecular interactions important in maintaining autoinhibition. Point mutations that are known to disrupt these interactions results in increased basal activity and membrane translocation. **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001 (Student's unpaired t test).