Abstract
Artificial cerebrospinal fluid (ACSF), i.e., brain extracellular medium, which includes Ca2+ and Mg2+, but not other divalent cations such as Zn2+, has been used for in vitro and in vivo experiments. The present study deals with the physiological significance of extracellular Zn2+ in ACSF. Spontaneous presynaptic activity is suppressed in the stratum lucidum of brain slices from young rats bathed in ACSF containing 10 nM ZnCl2, indicating that extracellular Zn2+ modifies hippocampal presynaptic activity. To examine the in vivo action of 10 nM ZnCl2 on long-term potentiation (LTP), the recording region was perfused using a recording electrode attached to a microdialysis probe. The magnitude of LTP was not modified in young rats by perfusion with ACSF containing 10 nM ZnCl2, compared to perfusion with ACSF without Zn2+, but attenuated by perfusion with ACSF containing 100 nM ZnCl2. Interestingly, the magnitude of LTP was not modified in aged rats even by perfusion with ACSF containing 100 nM ZnCl2, but enhanced by perfusion with ACSF containing 10 mM CaEDTA, an extracellular Zn2+ chelator. The present study indicates that the basal levels of extracellular Zn2+, which are in the range of low nanomolar concentrations, are critical for synaptic activity and perhaps increased age-dependently.
Divalent cations such as Ca2+ and Mg2+ play key roles for synaptic neurotransmission1. Among divalent cations, Ca2+ concentration is the highest in the brain and is approximately 1.2 mM in the cerebrospinal fluid (CSF) and brain extracellular fluid in the adult rats2. The influx of extracellular Ca2+ into neurons is essential for strengthening of synaptic connections between neurons, i.e., synaptic plasticity such as long-term potentiation (LTP)3,4. Approximately 2 mM Ca2+ is added to conventional artificial cerebrospinal fluid (ACSF) based on essentiality of intracellular Ca2+ signaling in neurons and glial cells, which is induced by the influx of extracellular Ca2+. In contrast, excess influx of extracellular Ca2+ into neurons leads to the pathophysiological process of neurodegeneration associated with glutamate excitotoxicity5,6,7.
Zinc serves as zinc metalloproteins and is also essential for synaptic neurotransmission8. Furthermore, a subclass of glutamatergic neurons is zincergic and is stained by Timm’s sulfide-silver method, which stains histochemically reactive zinc in the presynaptic vesicles. Zn2+ is co-released with glutamate in activity-dependent manner9 and play a role as a signal factor as well as Ca2+ 10. It has been reported that synaptic Zn2+ signaling is critical for LTP induction in the hippocampus. At mossy fiber-CA3 pyramidal cell synapses and Schaffer collateral-CA1 pyramidal cell synapses, which are zincergic, extracellular Zn2+, which is increased by LTP induction, serves for intracellular Zn2+ signaling and is involved in learning and memory11,12,13,14. Even at medial perforant pathway-dentate granule cell synapses, which are non-zincergic, intracellular Zn2+ signaling, which originates in the internal stores containing Zn2+, is involved in memory acquisition and retention15,16.
Zn2+ concentration in the brain extracellular fluid, which is estimated to be approximately 10 nM in the adults17, is much lower than Ca2+ concentration. Thus, much less attention has been paid to essentiality of Zn2+ in brain extracellular fluid for synaptic function. ACSF, i.e., brain extracellular medium, without Zn2+ has been used for in vitro and in vivo experiments. It is possible that not only neuronal excitation but also synaptic plasticity such as LTP is modified in brain slices bathed in ACSF without Zn2+, in which original physiology might not appear18. Clarifying the action of extracellular Zn2+ in the range of physiological concentrations is important to understand synaptic function precisely and also to understand the bidirectional action of Zn2+ under physiological and pathological conditions. Low nanomolar concentrations of Zn2+ are more physiologically relevant than micromolar concentrations of Zn2+ widely used, which are often neurotoxic. To assess the physiological range of extracellular Zn2+ concentration, in the present study, the action of low nanomolar concentrations of Zn2+ added to ACSF was examined in both in vitro and in vivo experiments using young and old rats.
Results
Spontaneous presynaptic activity
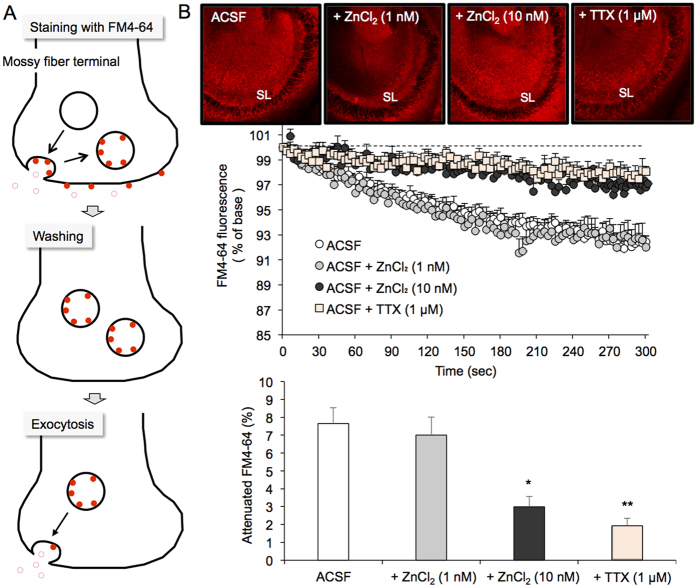
Spontaneous presynaptic activity, which was determined by the attenuation of FM4-64 fluorescence, was assessed in the stratum lucidum containing CA3 mossy fiber boutons of slices bathed in agents in ACSF (Fig. 1). FM4-64 fluorescence intensity was almost the same among four groups at the start of observation (time 0 sec) (upper images in Fig. 1B). Presynaptic activity in brain slices bathed in ACSF without Zn2+ was significantly higher than that in brain slices bathed in 10 nM ZnCl2 in ACSF, which was comparable with presynaptic activity in brain slices bathed in ACSF without Zn2+ under inhibition of neuronal depolarization in the presence of TTX (Fig. 1B). Presynaptic activity in brain slices bathed in 1 nM ZnCl2 was almost the same as that bathed in ACSF without Zn2+.
Figure 1. Suppression of spontaneous mossy fiber exocytosis of young rats in the presence of 10 nM ZnCl2.
(A) Schematic illustration of mossy fiber exocytosis assessed by attenuation of FM4-64 fluorescence. (B) FM4-64 fluorescence images in the CA3 after loading FM4-64 into slices bathed in ACSF, ACSF containing 1 nM Zn2+, and ACSF containing 10 nM Zn2+, and FM4-64 fluorescence image in the CA3 after loading of FM4-64 into slices bathed in ACSF and transferring the slices to ACSF containing 1 μM TTX (time 0 sec) (upper). Each point and line (mean ± SEM) represents the changes in FM4-64 fluorescence in the stratum lucidum (SL) of brain slices bathed in ACSF (n = 17), 1 nM ZnCl2 (n = 5), 10 nM ZnCl2 (n = 8) and 1 μM TTX (n = 6) after loading FM4-64 (time 0), which is expressed as 100%. (n = 6) (middle). Each bar and line (the mean ± SEM) represents the rate of the decreased FM4-64 fluorescence at time 300 sec (lower). *p < 0.05; **p < 0.01, vs. ACSF (Tukey’s test).
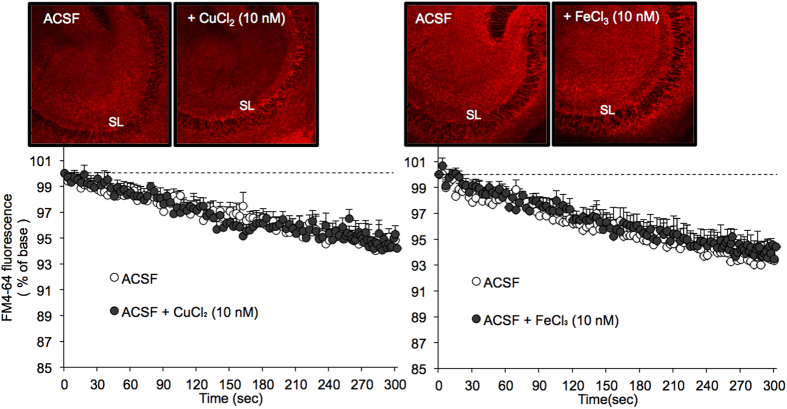
FM4-64 fluorescence intensity was almost the same between slices bathed in ACSF and ACSF containing 10 nM CuCl2 or ACSF containing 10 nM FeCl3 at the start of observation (time 0 sec) (upper images in Fig. 2). Presynaptic activity in brain slices was not modified by bathing in 10 nM CuCl2 or 10 nM FeCl3 in ACSF (Fig. 2).
Figure 2. No effect of 10 nM CuCl2 and 10 nM FeCl3 on spontaneous mossy fiber exocytosis of young rats.
FM4-64 fluorescence images in the CA3 after loading FM4-64 into slices bathed in ACSF and ACSF containing 10 nM Cu2+ or ACSF containing 10 nM Fe3+ (time 0 sec) (upper). Each point and line (mean ± SEM) represents the changes in FM4-64 fluorescence in the stratum lucidum (SL) of brain slices bathed in ACSF (n = 10), 10 nM CuCl2 (n = 6), and 10 nM FeCl3 (n = 6) after loading FM4-64 (time 0), which is expressed as 100%. (n = 6) (lower).
Intracellular levels of Ca2+ in the hippocampus
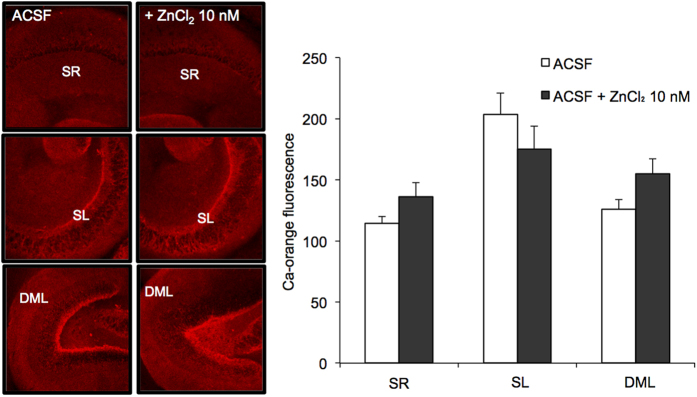
Variations in the intracellular levels of Ca2+ were compared between brain slices prepared with conventional ACSF without Zn2+ and bathed in ACSF containing 10 nM ZnCl2. Intracellular Ca2+ levels determined with calcium orange were also almost the same in the stratum radiatum, stratum lucidum and dentate molecular layer between the two groups (Fig. 3).
Figure 3. Intracellular Ca2+ imaging in the hippocampus of young rats with calcium orange AM.
Intracellular calcium orange fluorescence was imaged to estimate the basal levels of cytosolic Ca2+ in the hippocampus of brain slices bathed in ACSF (n = 7) and 10 nM ZnCl2 in ACSF (n = 7). SR, stratum radiatum; SL, stratum lucidum; DML. dentate molecular layer (left). Each bar and line (the mean ± SEM) represents fluorescent intensity in the SR, SL, and DML.
In vivo dentate gyrus LTP
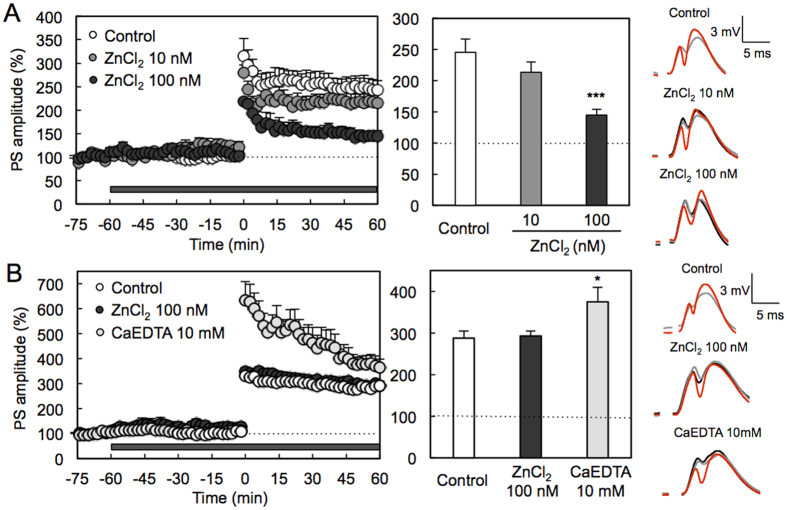
To pursue physiological range of extracellular Zn2+ concentration, in vivo LTP induction was compared between local perfusion of the recording area with ACSF without Zn2+ and ACSF containing Zn2+. LTP was not attenuated under perfusion with 10 nM ZnCl2, but significantly attenuated under perfusion with 100 nM ZnCl2 (Fig. 4A).
Figure 4. Action of extracellular Zn2+ and extracellular Zn2+ chelator in dentate gyrus LTP of young and aged rats.
(A) Recording region was perfused with ACSF in young rats and then 10 nM (n = 5) and 100 nM (n = 4) ZnCl2 in ACSF as shown by the shaded bar and HFS (10 trains of 20 pulses at 200 Hz separated by 1 s) was delivered at time 0 min (left). Each bar and line (mean ± SEM) represents the averaged PS amplitude of the last 10 min (time 50–60 min) (middle). ***p < 0.001, vs. control (ACSF) (n = 10) (Tukey’s test). Representative fEPSP recordings at time −70 (black line), −30 (grey line) and 50–60 min (red line) are shown (right). (B) Recording region was perfused with ACSF in aged rats and then 100 nM (n = 18) and 10 mM CaEDTA (n = 7) ZnCl2 in ACSF as shown by the shaded bar and HFS (10 trains of 20 pulses at 200 Hz separated by 1 s) was delivered at time 0 min (left). Each bar and line (mean ± SEM) represents the averaged PS amplitude of the last 10 min (time 50–60 min) (middle). *p < 0.05, vs. control (ACSF) (n = 26) (Tukey’s test). Representative fEPSP recordings at time −70 (black line), −30 (grey line) and 50–60 min (red line) are shown (right).
Adlard et al.19 report that metal chaperones for zinc and cupper, i.e., clioquinol and PBT2, prevent normal age-related cognitive decline and demonstrate that the metal chaperones are effective for preventing the zinc-mediated cognitive decline that is observed in aging and diseases, suggesting that extracellular Zn2+ concentration in the hippocampus is potentially changed along with aging. When LTP was induced under perfusion with 100 nM ZnCl2 in aged rats, it was not attenuated unlike the case of young rats (Fig. 4A and B). Interestingly, LTP was significantly enhanced under perfusion with 10 mM CaEDTA, an extracellular Zn2+ chelator, in aged rats (Fig. 4B), although no effect of 10 mM CaEDTA on LTP is reported in young rats15.
Discussion
Zn2+ -deficient ACSF has a negative impact on in vitro brain slice preparation and experiments. Hippocampal excitability is attenuated in brain slices pretreated with ACSF containing 20 nM ZnC12 for 1 h, compared with those pretreated with conventional ACSF without Zn2+ 20. An opposite effect on extracellular Zn2+ is observed between in vitro and in vivo LTP induction; in vitro CA1 LTP is enhanced in hippocampal slices bathed in 5 μM ZnC12 21,22, while in vivo CA1 LTP is attenuated under local perfusion of the recording region with 0.1–1 μM ZnCl2 by using a recording electrode attached to a microdialysis probe14. The evidence suggests that original synaptic activity is modified in slice experiments using ACSF without Zn2+ and that addition of Zn2+ to ACSF is necessary to prevent modification of synaptic function.
To clarify the physiological range of extracellular Zn2+ concentration, the action of low nanomolar concentrations of Zn2+ in ACSF was examined in both in vitro and in vivo experiments. On the basis of the estimated concentration of extracellular Zn2+ under the basal (static) condition, which is approximately 10 nM17, the present study performed focused on the concentration of 10 nM. Spontaneous presynaptic activity assessed with FM4-64 is significantly suppressed in the stratum lucidum of brain slices from young rats bathed in ACSF containing 10 nM Zn2+, but not in ACSF containing 10 nM Cu2+ or 10 nM Fe3+, indicating that hippocampal presynaptic activity is enhanced in brain slices prepared with ACSF without Zn2+. Micromolar Zn2+ also suppresses hippocampal mossy fiber exocytosis23. It is likely that Zn2+ dose-dependently suppresses presynaptic activity in the hippocampus. On the other hand, the basal levels of intracellular Ca2+ determined with calcium orange were not modified in the hippocampus of brain slices prepared with ACSF without Zn2+. Suh et al.24 report that acute brain slice preparations are poorly suitable for research on roles of endogenous Zn2+ released from zincergic neurons. Vesicular zinc determined by Timm’s sulfide-silver method is lost during slice preparation and slice incubation; in vitro Zn2+ release is reduced to about 25% of in vivo Zn2+ release. It is likely that extracellular Zn2+ is physiologically critical in the range of low nonomolar concentrations for in vitro slice experiments from young animals.
To examine the action of 10 nM Zn2+ on in vivo LTP induction, the recording region was perfused using a recording electrode attached to a microdialysis probe. The magnitude of LTP was not significantly modified in young rats by perfusion with ACSF containing 10 nM Zn2+, compared to perfusion with ACSF without Zn2+, but attenuated by perfusion with ACSF containing 100 nM Zn2+. Because 100 nM Zn2+ also attenuates CA1 LTP14, this concentration of Zn2+ is beyond the range of physiological concentration in young rats as the basal concentration of extracellular Zn2+. The present study indicates that extracellular Zn2+ modulates LTP at low nanomolar concentrations in young rats. Interestingly, the magnitude of LTP was not modified in aged rats by perfusion with ACSF containing 100 nM Zn2+. The perfusate reaches the equilibrium state with the brain extracellular fluid containing Zn2+, resulting in the perfusate (ACSF) with Zn2+. It is possible that Zn2+ concentration in the perfusate during the perfusion is higher in aged rats than in young rats. The magnitude of LTP was enhanced in aged rats by perfusion with ACSF containing 10 mM CaEDTA, while it is not modified in young rats under the same condition15. It is likely that extracellular Zn2+ concentration is increased age-dependently and that 100 nM Zn2+ is in the range of physiological concentration in aged rats. Extracellular Zn2+ may suppressively modulate LTP induction, especially in aged rats.
Zinc concentration in the CSF is reported to be 150–380 nM25,26,27. If it is the same as zinc concentration in the brain extracellular fluid, a large portion of zinc is not free ion in the brain extracellular fluid under the basal condition. At zincergic synapses, extracellular Zn2+ levels are dynamically changed by the degree of Zn2+ activity-dependently released from neuron terminals, which is required for learning and memory via synaptic plasticity14. Even at non-zincergic synapses, extracellular Zn2+ may serve as a pool for Zn2+ release from the internal stores, which is also required for learning and memory15. In conclusion, the present study indicates that original neurophysiology may not appear in ACSF without Zn2+. The basal levels of extracellular Zn2+, which are in the range of low nanomolar concentrations, are critical for synaptic activity and perhaps increased age-dependently. Homeostasis of Zn2+ in both extracellular and intracellular compartments is still poorly understood and the understanding is important to search a strategy for preventing Zn2+ -mediated cognitive decline.
Experimental Procedures
Chemicals and animals
Male Wistar rats were purchased from Japan SLC (Hamamatsu, Japan) and used as young (6–9 weeks of age) and aged (>60 weeks of age) rats. They were housed under the standard laboratory conditions (23 ± 1 °C, 55 ± 5% humidity) and had access to tap water and food ad libitum. FM4–64, an indicator of presynaptic activity, and calcium orange AM, a membrane-permeable calcium indicator, were purchased from Sigma-Aldrich (St. Louis, MO) and Molecular Probes, Inc. (Eugene, OR), respectively. These indicators were dissolved in dimethyl sulfoxide (DMSO) and then diluted to artificial cerebrospinal fluid (ACSF) containing 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 1.0 mM NaH2PO4, 2.5 mM CaCl2, 26.2 mM NaHCO3, and 11 mM D-glucose (pH 7.3). ZnCl2, FeCl3 and CuCl2 were dissolved in purified water and prepared as 10 mM stock solutions. The stock solutions were diluted to 1 μM with purified water. One micromolar metals in water were diluted with ACSF and nanomolar solutions were freshly prepared.
Brain slice preparation
Mice were anesthetized with ether and decapitated. The brain was quickly removed and immersed in ice-cold choline-ACSF containing 124 mM choline chloride, 2.5 mM KCl, 2.5 mM MgCl2, 1.25 mM NaH2PO4, 0.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose (pH 7.3) to suppress excessive neuronal excitation. Horizontal brain slices (400 μm) were prepared by using a vibratome ZERO-1 (Dosaka Kyoto, Japan) in an ice-cold choline-ACSF. Slices were then maintained in ACSF and ACSF containing 1–10 nM ZnCl2, 10 nM CuCl2, or 10 nM FeCl3 at 25 °C for 1 h. All solutions used in the experiments were continuously bubbled with 95% O2 and 5% CO2.
Spontaneous exocytosis
The brain slices bathed in ACSF and ACSF containing Zn2+, Cu2+, or Fe3+ were transferred to an incubation chamber filled with each ACSF containing 5 μM FM4-64, 45 mM KCl, and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an antagonist of AMPA/kainate receptors, allowed to stand at 25 °C for 90 s and transferred a chamber filled with each ACSF to wash out extracellular FM4-64 and KCl for 15 min. Brain slices were transferred to a recording chamber filled with each ACSF containing 10 μM CNQX to prevent recurrent activity. FM4-64 fluorescence (excitation, 543 nm; emission, 640 nm) was measured with a confocal laser-scanning microscopic system LSM 510 META at the rate of 1 Hz for 300 s through a 10 × objective. In another experiment, the brain slices bathed in ACSF was treated in the same manner for staining with FM4-64 and transferred to a recording chamber filled with 1 μM tetrodotoxin (TTX), a voltage-gated sodium channel blocker, in ACSF containing 10 μM CNQX to measure the change in FM4-64 fluorescence under inhibition of neuronal depolarization. Because FM4-64 fluorescence originates in vesicular membrane-bound FM4-64, FM4-64 fluorescence is attenuated by spontaneous presynaptic activity28,29 (Fig. 1A). FM4-64 fluorescence was then normalized by the initial fluorescence intensity at the time 0 sec, which is expressed as 100%. The rate (%) of attenuated FM4-64 fluorescence at the time 300 sec was compared among groups bathed in ACSF and reagents in ACSF.
Intracellular levels of Ca2+
To assess the basal levels of intracellular Ca2+, the brain slices in ACSF and ACSF containing 10 nM ZnCl2 were placed for 30 min in each ACSF containing 5 μM calcium orange AM, transferred to a chamber filled with each ACSF to wash out extracellular calcium orange AM for 15 min, and transferred to a recording chamber filled with each ACSF. The fluorescence of calcium orange (excitation, 543 nm; monitoring, above 560 nm) was measured with a confocal laser-scanning microscopic system LSM 510 META for 30 sec at the rate of 1 Hz through a 10 × objective.
In vivo LTP
Male rats were anesthetized with chloral hydrate (400 mg/kg) and placed in a stereotaxic apparatus. A bipolar stimulating electrode and a monopolar recording electrode made of tungsten wire attached to an microdialysis probe (E-A-I-12-01, Eicom Co., Kyoto) were positioned stereotaxically so as to selectively stimulate the perforant pathway while recording in the dentate gyrus under local perfusion with agents in ACSF (127 mM NaCl, 2.5 mM KCl, 0.9 mM MgCl2, 1.2 mM NaH2PO4, 1.3 mM CaCl2, 21 mM NaHCO3, and 3.4 mM D-glucose (pH 7.3)). The electrode stimulating the perforant pathway was positioned 8.0 mm posterior to the bregma, 4.5 mm lateral, 3.0–3.5 mm inferior to the dura. A recording electrode was implanted ipsilaterally 4.0 mm posterior to the bregma, 2.3–2.5 mm lateral and 3.0–3.5 mm inferior to the dura. All the stimuli were biphasic square wave pulses (200 μs width) and their intensities were set at the current that evoked 40% of the maximum population spike (PS) amplitude. Test stimuli (0.05 Hz) were delivered at 20 s intervals to monitor PS amplitude. At the beginning of the experiments, input/output curves were generated by systematic variation of the stimulus current (0.1–5.0 mA) to evaluate synaptic potency. After stable baseline recording under perfusion with ACSF for at least 30 min, PS amplitudes were measured under perfusion with agents in ACSF for 60 min and LTP was induced by delivery of high-frequency stimulation (HFS; 10 trains of 20 pulses at 200 Hz separated by 1 s) and recorded for 60 min. PS amplitudes (test frequency: 0.05 Hz) were averaged over 120-second intervals and expressed as percentages of the mean PS amplitude measured during the 15-min baseline period (from −75 min ~ to −60 min) perfused with ACSF prior to LTP induction, which was expressed as 100%. PS amplitudes for the last 10 min were also averaged and represented as the magnitude of LTP.
Statistical analysis
For statistical analysis, Student’s t-test was used for comparison of the means of unpaired or paired two-data. For multiple comparisons, differences between the control and treatments were assessed by one-way ANOVA followed by post hoc testing using the Tukey’s test (the statistical software, GraphPad Prism 5). A value of p < 0.05 was considered significant. Data were expressed as means ± standard error. The results of statistical analysis are described in each figure legend. In Figs 1, 2 and 3, “n” means the number of slices. The experiments have separately done in triplicate to confirm the reproducibility and all slices used in the present study were analyzed statistically. In Fig. 4, “n” means the number of rats.
Ethics Statement
All experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the University of Shizuoka that refer to American Association for Laboratory Animals Science and the guidelines laid down by the NIH (NIH Guide for the Care and Use of Laboratory Animals) in the USA. The ethics committee of the University of Shizuoka has approved all experimental protocols (The approval number, 136043).
Additional Information
How to cite this article: Tamano, H. et al. In vitro and in vivo physiology of low nanomolar concentrations of Zn2+ in artificial cerebrospinal fluid. Sci. Rep. 7, 42897; doi: 10.1038/srep42897 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
The authors declare no competing financial interests.
Author Contributions Planned experiments: H.T., and A.T. Performed the Experiments: R.N., Y.S., M.S., Y.K. and M.O. Analyzed data: Y.S., H.T. and A.T. Wrote the paper: H.T., and A.T. All authors reviewed the manuscript.
References
- Kim N. K. & Robinson H. P. Effects of divalent cations on slow unblock of native NMDA receptors in mouse neocortical pyramidal neurons. Eur. J. Neurosci. 34, 199–212 (2011). [DOI] [PubMed] [Google Scholar]
- Jones H. C. & Keep R. F. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J. Physiol. 402, 579–593 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G., Cooke S. F. & Bliss T. V. P. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 9, 65–67 (2008). [DOI] [PubMed] [Google Scholar]
- Lisman J., Yasuda R. & Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E. et al. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47, 264–272 (2010). [DOI] [PubMed] [Google Scholar]
- Bickler P. E. et al. Anesthetic protection of neurons injured by hypothermia and rewarming: roles of intracellular Ca2+ and excitotoxicity. Anesthesiology 117, 280–292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abushik P. A., Sibarov D. A., Eaton M. J., Skatchkov S. N. & Antonov S. M. Kainate-induced calcium overload of cortical neurons in vitro: Dependence on expression of AMPAR GluA2-subunit and down-regulation by subnanomolar ouabain. Cell Calcium 54, 95–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstead H. H. Subclinical zinc deficiency impairs human brain function. J. Trace Elem. Med. Biol. 26, 70–73 (2012). [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Koh J. Y. & Bush A. I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449–462 (2005). [DOI] [PubMed] [Google Scholar]
- Nakashima A. S. & Dyck R. H. Zinc and cortical plasticity. Brain Res. Rev. 59, 347–373 (2009). [DOI] [PubMed] [Google Scholar]
- Li Y., Hough C. J., Frederickson C. J. & Sarvey J. M. Induction of mossy fiber – >Ca3 long-term potentiation requires translocation of synaptically released Zn2+. J. Neurosci. 21, 8015–8025 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Z., Pan E., Xiong Z. Q. & McNamara J. O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546–558 (2008). [DOI] [PubMed] [Google Scholar]
- Pan E. et al. Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3 synapse. Neuron 71, 1116–1126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Suzuki M., Tempaku M., Ohashi K. & Tamano H. Influx of extracellular Zn2+ into the hippocampal CA1 neurons is required for cognitive performance via long-term potentiation. Neuroscience 304, 209–216 (2015). [DOI] [PubMed] [Google Scholar]
- Takeda A. et al. Intracellular Zn2+ signaling in the dentate gyrus is required for object recognition memory. Hippocampus 24, 1404–1412 (2014). [DOI] [PubMed] [Google Scholar]
- Tamano H. et al. Blockade of intracellular Zn2+ signaling in the dentate gyrus erases recognition memory via impairment of maintained LTP. Hippocampus 25, 952–962 (2015). [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp. Neurol. 198, 285–293 (2006). [DOI] [PubMed] [Google Scholar]
- Takeda A. & Tamano H. Significance of low nanomolar concentration of Zn2+ in artificial cerebrospinal fluid. Mol. Neurobiol. PMID: 26984599 (2016). [DOI] [PubMed] [Google Scholar]
- Adlard P. A. et al. Metal chaperones prevent zinc-mediated cognitive decline. Neurobiol. Dis. 81, 196–202 (2015). [DOI] [PubMed] [Google Scholar]
- Tamano H. et al. Preventive effect of 3,5-dihydroxy-4-methoxybenzyl alcohol and zinc, Pacific oyster components, on glutamatergic neuron activity in the hippocampus. Biol. Bul. 229, 282–288 (2015). [DOI] [PubMed] [Google Scholar]
- Takeda A., Fuke S., Ando M. & Oku N. Positive modulation of long-term potentiation at hippocampal CA1 synapses by low micromolar concentrations of zinc. Neuroscience 158, 585–591 (2009). [DOI] [PubMed] [Google Scholar]
- Lorca R. A. et al. Zinc enhances long-term potentiation through P2X receptor modulation in the hippocampal CA1 region. Eur. J. Neurosci. 33, 1175–1185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A. et al. Inhibition of presynaptic activity by zinc released from mossy fiber terminals during tetanic stimulation. J. Neurosci. Res. 83, 167–176 (2006). [DOI] [PubMed] [Google Scholar]
- Suh S. W. et al. Release of synaptic zinc is substantially depressed by conventional brain slice preparations. Brain Res. 879, 7–12 (2000). [DOI] [PubMed] [Google Scholar]
- Hershey C. O. et al. Cerebrospinal fluid trace element content in dementia: clinical, radiologic, and pathologic correlations. Neurology 33, 1350–1353 (1983). [DOI] [PubMed] [Google Scholar]
- Gellein K. et al. Trace elements in cerebrospinal fluid and blood from patients with a rare progressive central and peripheral demyelinating disease. J. Neurol. Sci. 266, 70–78 (2008). [DOI] [PubMed] [Google Scholar]
- Michalke B. & Nischwitz V. Review on metal speciation analysis in cerebrospinal fluid-current methods and results: a review. Anal. Chim. Acta 682, 23–36 (2010). [DOI] [PubMed] [Google Scholar]
- Klingauf J., Kavalali E. T. & Tsien R. W. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature 394, 581–585 (1998). [DOI] [PubMed] [Google Scholar]
- Zakharenko S. S., Zablow L. & Siegelbaum S. A. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat. Neurosci. 4, 711–717 (2001). [DOI] [PubMed] [Google Scholar]