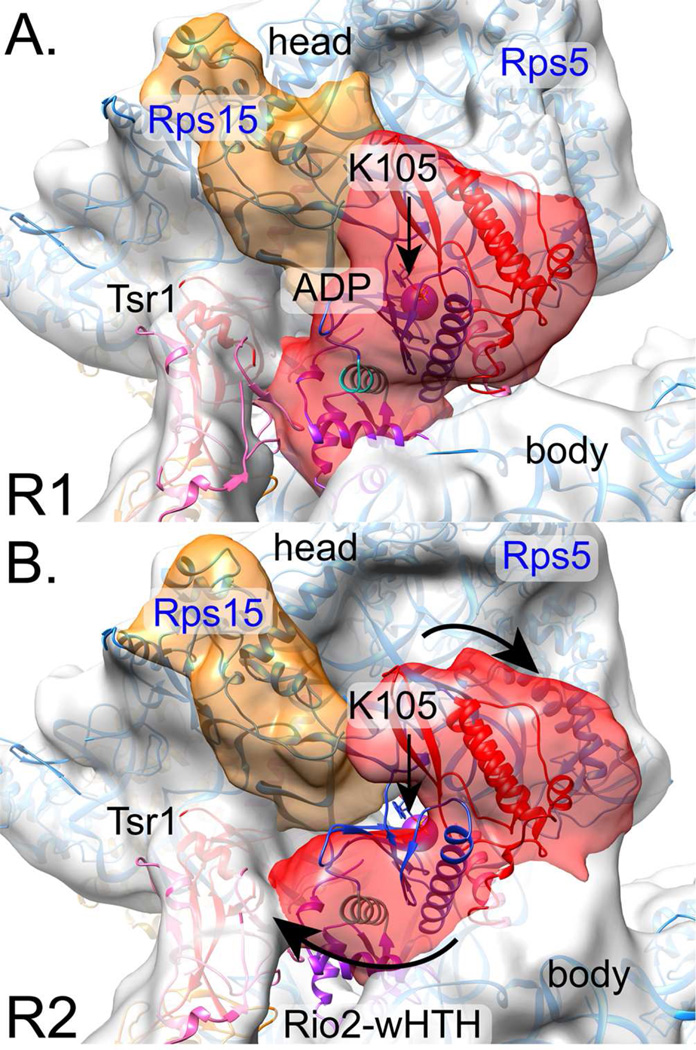
Fig. 3. Rio2 adopts multiple conformations, bound between the head and the body.
A. Subclass R1 shows Rio2 as an extended, wedge-shaped density (red), with a small lobe close to Tsr1IV and a larger lobe bridging the head and body. The X-ray crystal structure of C. thermophilum Rio2 homolog (PDB 4GYG, (Ferreira-Cerca et al., 2012)) fits well, in light of C-terminal sequence differences between ctRio2 and scRio2. Rio2 is colored per Ferreira-Cerca et al., 2012: the N-terminal wHTH is purple, the N lobe is blue, the C-lobe is in red, and two linker helices are turquoise or pink. A purple sphere represents K105’s Cα atom position.
B. Subclass R2 reveals density corresponding to Rio2 as a U-shape (red) into which the X-ray crystal structure of ctRio2 does not fit well. To fit the density, the wHTH would need to rotate towards Tsr1IV, while the C-lobe would need to rotate away from its central ATP binding pocket and towards the pre-40S body. Arrows demark these suggested movements. See also Figure S2.