Abstract
Traffic-related air pollution (TRAP) may affect immune responses, including those in the TH2 and TH17 pathways. To examine whether TRAP is associated with plasma level of TH17-, TH1-, and TH2-related cytokines in children with and without asthma, a cross-sectional study of 577 children (ages 6–14 years) with (n = 294) and without (n = 283) asthma in San Juan (Puerto Rico) was performed. Residential distance to a major road was estimated using geocoded home addresses for study participants. A panel of 14 cytokines, enriched for the TH17 pathway, was measured in plasma. Asthma was defined as physician-diagnosed asthma and current wheeze. Multivariable linear regression was used to examine the association of residential distance to a major road (a marker of TRAP), asthma, and cytokine levels. Among all participating children, residential proximity to a major road was significantly associated with increased plasma level of IL-31, even after adjustment for relevant covariates and correction for multiple testing. The presence of asthma modified the estimated effect of the residential distance to a major road on plasma TNF-α (P for interaction = 0.00047). Although living farther from a major road was significantly associated with lower TNF-α level in control subjects, no such decrease was seen in children with asthma. In a direct comparison of cases and control subjects, children with asthma had significantly higher levels of IL-1β, IL-22, and IL-33 than control subjects. TRAP is associated with increased levels of proinflammatory cytokines among Puerto Rican children, who belong to an ethnic group with high risk for asthma.
Introduction
Asthma is the most common chronic respiratory disease of childhood in the United States, where ∼7 million children are affected.1 Traffic-related air pollution (TRAP) has been implicated in the pathogenesis of childhood asthma.2,3 Moreover, TRAP has been associated with eosinophilic inflammation and TH2 immune responses,4 as well as neutrophilic inflammation,5 TH17 cell differentiation,6 and increased IL-17 serum levels.7
Proximity to a major road, a proxy for TRAP, is associated with asthma and morbidity from asthma in Puerto Rican children.3,8 To further understand the potential effects of TRAP on immune responses in Puerto Rican children, we examined the relation between proximity to a major road and plasma cytokines (enriched for the TH17 pathway) in a study of 577 children with (cases) and without (control subjects) asthma in San Juan (Puerto Rico). Moreover, we compared plasma cytokine levels between cases and control subjects.
Methods
Subject recruitment
From March 2009 to June 2010, children in San Juan were recruited from randomly selected households, as previously described.9 In brief, households were selected using a multistage probability sample design. Primary sampling units (PSUs) were randomly selected neighborhood clusters based on the 2000 US Census, and secondary sampling units were randomly selected households within each PSU. On the basis of this design, 7,073 households were selected, and 6,401 (90.5%) were contacted: 1,111 households had one or more children who met inclusion criteria (age 6 to 14 years, 4 Puerto Rican grandparents, and residence in the same household for at least 1 year). Of these 1,111 households, 438 (39.4%) had at least 1 child with asthma (a case, defined as having physician-diagnosed asthma and at least 1 episode of wheeze in the prior year). From these 438 households, 1 child with asthma was selected (at random if there was more than 1 such child). Similarly, only 1 child without asthma (a control subject, having neither physician-diagnosed asthma nor wheeze in the prior year) was randomly selected from the remaining 673 households. To reach our target sample size (n = 700),9 we attempted to enroll 783 of the 1,111 eligible children. Parents of 105 (13.4%) of these 783 children refused to participate or could not be reached, leaving 678 participants (351 cases and 327 control subjects). There were no significant differences in age, gender, or area of residence between eligible children who did (n = 678) and did not (n = 105) agree to participate. Of the 678 study participants, 578 (85.1%) had blood samples for plasma cytokine measurements; 577 of these 578 participants also had data on their home address, and were thus included in the current analysis.
Study procedures
Study participants completed a protocol that included questionnaires and collection of blood samples. One of the child's caretakers [usually (∼93%) the mother] completed a questionnaire that was slightly modified from one used in the Collaborative Study of the Genetics of Asthma.10 Residential distance from the home address of each participant to a major roadway was geocoded (linked) to a 15-digit 2000 US Census Federal Information Processing Standard (FIPS) + block code at the University of Puerto Rico, as previously described.8 Using the program ArcMAP10.1 (ArcGIS 10.1; Esri, Redlands, CA), centroids were then created by obtaining X and Y coordinates for the center of individual census blocks based on a 2000 US Census map for Puerto Rico. The distance from the residential block centroid of a participant to the nearest major road (defined by Esri 2012 Data and Maps major road layer) was then calculated using the geoprocessing proximity tool “near,” which measures the nearest distance “as the crow flies” between 2 features.
A panel of 14 cytokines [enriched for the TH17 pathway, and including interleukin (IL)-1β, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, IL-31, IL-33, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α], was measured in plasma samples using the Bio-Plex Pro Human TH17 Cytokine Panel on the Bio-Plex HTF system (Bio-Rad Laboratories, Inc., Hercules, CA) as per the manufacturer's instructions, with all samples measured in duplicate. After assigning a small constant [half the lowest detectable level (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ped)] to nondetectable levels, plasma cytokine levels were log10 transformed for data analysis, which led to an approximately normal distribution for each cytokine.
Written parental consent and child's assent was obtained for participants in the study, which was approved by the Institutional Review Boards of the University of Puerto Rico (San Juan, PR), Brigham and Women's Hospital (Boston, MA), and the University of Pittsburgh (Pittsburgh, PA).
Statistical analysis
Our outcome of interest was log10 transformed plasma cytokine levels. Our exposure of interest was the residential distance to a major road. The primary analysis was conducted in all study participants [including control subjects and subjects with asthma (defined as physician-diagnosed asthma and ≥1 episode of wheeze in the previous year)].
The following covariates were compared between cases and controls in unadjusted analyses: age, gender, annual household income [< vs. ≥$15,000 (near the median household income for Puerto Rico in 2008–2009)], type of health insurance (private or employer-based vs. others), body mass index (BMI)11 as a z-score (based on CDC growth charts12), current environmental tobacco smoke (ETS),13 and ETS in early life (in utero or in the first 2 years). The characteristics of children with asthma were compared with those of control subjects using chi square tests for pairs of binary variables, and 2-tailed t-tests or Wilcoxon rank-sum test for pairs of binary and continuous variables, as appropriate.
The correlation within each pair of cytokines was tested using a Pearson correlation coefficient. Correlations that were ≥0.60 and were significant at P < 0.01 were considered strong enough to count as “the same cytokine” for purposes of a modified Bonferroni correction for multiple testing, an approach similar to one widely used to account for correlation among genetic variants in association studies14 (eg, if 9 pairs met these criteria, the number of effective tests would be 14 − 9 = 5).
Ordinal logistic regression was used for the unadjusted analysis of quartiles of residential distance to a major road and plasma cytokine levels. Linear regression was then used for the multivariable analysis of residential distance to a major road and plasma cytokine levels, adjusting for age, gender, annual household income, current ETS exposure, and asthma (case–control status). For each model, we also tested for a first-order interaction between residential distance to a roadway and asthma. If such interaction was nominally significant (P < 0.05), the analysis was conducted separately in cases and control subjects (eg, after stratifying by asthma).
Linear regression was then used to compare plasma cytokine levels between cases and control subjects, adjusting for age, gender, and annual household income. All statistical analyses were performed with SAS software (version 9.4; SAS Institute, Cary, NC).
Results
The main characteristics of the 577 participating children are shown in Table 1. Compared with control subjects, children with asthma were significantly more likely to be male and to be exposed to current or early life ETS. There were no significant differences in age, residential distance to a major road, BMI z-score, household income, or type of health insurance between children with asthma and control subjects.
Table 1.
Main Characteristics of Study Participants
| Variables | Controls (n = 283) | Asthma (n = 294) | All children (n = 577) |
|---|---|---|---|
| Age, years | 10.5 (2.8) | 10.1 (2.6) | 10.3 (2.7) |
| Male gender | 136 (48) | 170 (58)a | 306 (53) |
| Distance from a major roadway, meters | 374 (297) | 333 (270) | 353 (284) |
| BMI, z-score | 0.50 (1.11) | 0.65 (1.18) | 0.57 (1.15) |
| Private or employer-based health insurance | 100 (35) | 88 (30) | 188 (33) |
| Annual household income >$15,000 | 94 (35) | 91 (32) | 185 (33) |
| Current ETS exposure | 102 (36) | 134 (46)a | 236 (41) |
| ETS exposure in utero or before age 2 years | 116 (41) | 148 (50)a | 264 (46) |
Results displayed as mean (SD) for continuous and n (%) for categorical variables.
P < 0.05 for comparison with control subjects.
BMI, body mass index; ETS, environmental tobacco smoke.
Supplementary Fig. S1 and Supplementary Table S2 show the pairwise correlations among the measured plasma cytokines. In this analysis, 9 pairs of cytokines had a correlation coefficient ≥0.60 and P < 0.01, as follows: IL-6 and IL-25, IL-6 and IFN-γ, IL-10 and IL-22, IL-10 and IL-25, IL-10 and IL-33, IL17F and IFN-γ, IL-25 and IL-31, IL-25 and IFN-γ, and IL-25 and TNF-α. Thus, we considered a P < 0.01 as significant after a Bonferroni correction for 5 independent tests (P = 0.05/5 = 0.01).
Table 2 shows the relation between quartiles of residential distance to a major road and plasma levels of each cytokine. In this analysis, distance to a major road was linearly associated with lower levels of 5 cytokines at P < 0.05: IL-4, IL-6, IL-10, IL-17F, and IL-31. After correction for multiple testing, the association was borderline statistically significant for IL-17F (P = 0.01) and statistically significant for IL-31 (P = 0.004).
Table 2.
Distance from a Major Roadway (in Quartiles) and Plasma Cytokine Levels in Study Participants (n = 577)
| Log10 (pg/mL) selected cytokine level | Quartile 1 (<172 m), n = 131 | Quartile 2 (172–220 m), n = 161 | Quartile 3 (221–496 m), n = 151 | Quartile 4 (>496 m), n = 134 | P for trend |
|---|---|---|---|---|---|
| IL 1β | −0.07 (0.42) | −0.19 (0.62) | −0.10 (0.57) | −0.21 (0.56) | 0.15 |
| IL 4 | 0.58 (0.93) | 0.71 (0.89) | 0.35 (0.97) | 0.46 (0.96) | 0.04 |
| IL 6 | 0.83 (0.46) | 0.79 (0.56) | 0.72 (0.58) | 0.71 (0.59) | 0.04 |
| IL 10 | 0.67 (1.01) | 0.64 (1.04) | 0.54 (0.97) | 0.41 (1.04) | 0.03 |
| IL 17A | 0.70 (0.84) | 0.48 (0.91) | 0.75 (0.80) | 0.59 (0.81) | 0.98 |
| IL 17F | 0.99 (1.06) | 1.23 (0.96) | 0.74 (1.08) | 0.82 (1.09) | 0.01 |
| IL 21 | 0.64 (0.77) | 0.58 (0.78) | 0.57 (0.74) | 0.47 (0.70) | 0.06 |
| IL 22 | 0.41 (0.91) | 0.25 (0.96) | 0.45 (0.93) | 0.30 (0.93) | 0.80 |
| IL 23 | 0.09 (0.59) | −0.01 (0.39) | 0.15 (0.62) | 0.09 (0.57) | 0.37 |
| IL 25 | 0.66 (0.32) | 0.66 (0.46) | 0.60 (0.37) | 0.58 (0.42) | 0.06 |
| IL 31 | 1.32 (0.87) | 1.49 (0.80) | 1.17 (1.00) | 1.11 (1.02) | 0.004 |
| IL 33 | 1.75 (0.60) | 1.58 (0.84) | 1.76 (0.53) | 1.61 (0.76) | 0.49 |
| IFN γ | 0.79 (1.08) | 1.02 (1.10) | 0.59 (1.12) | 0.69 (1.10) | 0.07 |
| TNFα | 0.85 (0.23) | 0.84 (0.23) | 0.83 (0.23) | 0.80 (0.27) | 0.13 |
Results presented as mean (SD).
P value for trend obtained using unadjusted ordinal regression.
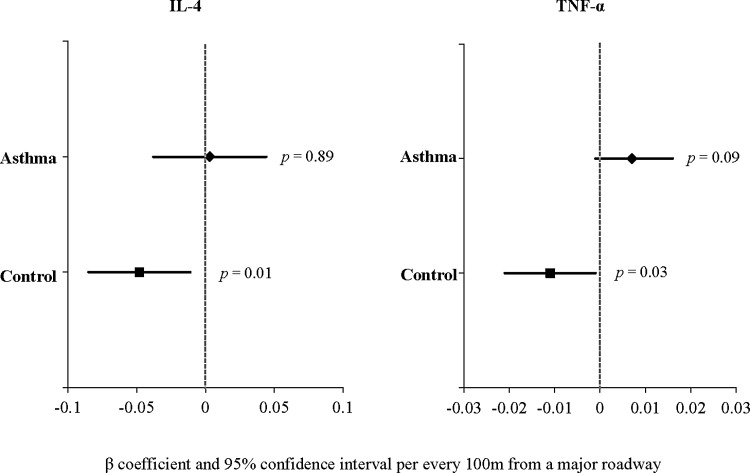
Table 3 shows the results of the linear regression analysis of residential distance to a major road and plasma cytokine levels, after adjustment for age, gender, annual household income, current ETS, and asthma (case–control status). In this analysis, distance to a major road remained significantly associated with lower level of IL-31 (P = 0.007). An interaction between residential distance to a road and case–control status (asthma) was significant for plasma levels of TNF-α (P = 0.0047), but not for any other plasma cytokine level. Thus, the analysis of TNF-α was stratified by the presence or absence of asthma (Fig. 1). In this stratified analysis, residential distance was associated with lower TNF-α in control subjects (β = −0.011, 95% CI = −0.021 to −0.001, P = 0.03), but not in cases (P = 0.08).
Table 3.
Linear Regression Analysis of Residential Distance to a Major Road and Plasma Cytokines in 577 Study Participants
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| Plasma cytokine level | β (95% CI) | P | β (95% CI) | P |
| IL-1β | −0.010 (−0.026 to 0.006) | 0.22 | −0.011 (−0.027 to 0.005) | 0.19 |
| IL-4 | −0.023 (−0.050 to 0.004) | 0.09 | −0.03 (−0.05 to 0.002) | 0.07 |
| IL-6 | −0.013 (−0.029 to 0.003) | 0.10 | −0.011 (−0.027 to 0.005) | 0.16 |
| IL-10 | −0.029 (−0.058 to 0.0002) | 0.05 | −0.030 (−0.059 to −0.0001) | 0.047 |
| IL-17A | 0.004 (−0.021 to 0.026) | 0.76 | 0.005 (−0.020 to 0.029) | 0.72 |
| IL-17F | −0.034 (−0.065 to −0.004) | 0.03 | −0.030 (−0.061 to 0.0004) | 0.05 |
| IL-21 | −0.016 (−0.037 to 0.006) | 0.15 | −0.016 (−0.038 to 0.006) | 0.15 |
| IL-22 | 0.0002 (−0.027 to 0.027) | 0.99 | 0.0002 (−0.027 to 0.028) | 0.99 |
| IL-23 | 0.010 (−0.006 to 0.026) | 0.22 | 0.012 (−0.004 to 0.028) | 0.14 |
| IL-25 | −0.010 (−0.021 to 0.002) | 0.10 | −0.008 (−0.020 to 0.004006) | 0.17 |
| IL-31 | −0.038 (−0.064 to −0.011) | 0.006 | −0.038 (−0.065 to −0.011) | 0.007 |
| IL-33 | −0.005 (−0.026 to 0.015) | 0.60 | −0.008 (−0.028 to 0.013) | 0.46 |
| IFN-γ | −0.031 (−0.063 to 0.001) | 0.06 | −0.025 (−0.057 to 0.007) | 0.13 |
| TNF-α | −0.005 (−0.011 to 0.002) | 0.19 | −0.011 (−0.02 to −0.002) | 0.01b |
Bold indicates statistical significance at alpha level 0.05.
Adjusted for age, gender, household income, current ETS exposure, and case–control status.
For TNF-α, P value is from main effects in models that also include the interaction term (interaction term P = 0.0047 for TNF- α; nonsignificant, and thus removed from the model, for all other cytokines).
FIG. 1.
Multivariate regression analysis of proximity to a major road and TNF-α, in control subjects and in children with asthma. All models were adjusted for age, gender, current environmental tobacco smoke exposure, and annual household income.
Because of strong co-linearity, we could not include both household income and type of health insurance in the multivariable models. However, we obtained very similar results when the multivariate models were adjusted for type of health insurance instead of household income (data not shown).
Finally, we compared plasma cytokine levels between children with and without asthma (Supplementary Table S3). Compared with control subjects, children with asthma had higher plasma levels of IL-1β, IL-17F, IL-22, and IL-33 (P < 0.01 in all instances). After adjustment for age, gender, and current ETS, these differences were nominally significant for IL-1β, IL-22, IL-31, and IL-33; after correction for multiple testing, all but IL-31 remained significant (Table 4).
Table 4.
Linear Regression Analysis of Plasma Cytokines and Asthma in 577 Study Participants
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| Plasma cytokine | OR (95% CI) | P | OR (95% CI) | P |
| IL-1β | 1.65 (1.21 to 2.26) | 0.001 | 1.63 (1.18 to 2.25) | 0.003 |
| IL-4 | 0.95 (0.80 to 1.13) | 0.56 | 0.98 (0.82 to 1.17) | 0.82 |
| IL-6 | 1.09 (0.81 to 1.47) | 0.56 | 1.23 (0.90 to 1.68) | 0.20 |
| IL-10 | 1.13 (0.96 to 1.33) | 0.13 | 1.18 (0.994 to 1.39) | 0.06 |
| IL-17A | 1.17 (0.97 to 1.42) | 0.11 | 1.17 (0.96 to 1.43) | 0.13 |
| IL-17F | 0.82 (0.71 to 0.96) | 0.01 | 0.86 (0.74 to 1.02) | 0.07 |
| IL-21 | 1.19 (0.96 to 1.48) | 0.12 | 1.22 (0.97 to 1.53) | 0.09 |
| IL-22 | 1.28 (1.08 to 1.53) | 0.006 | 1.32 (1.10 to 1.58) | 0.003 |
| IL-23 | 0.83 (0.62 to 1.13) | 0.23 | 0.84 (0.61 to 1.14) | 0.26 |
| IL-25 | 1.05 (0.70 to 1.57) | 0.83 | 1.19 (0.78 to 1.82) | 0.41 |
| IL-31 | 1.16 (0.97 to 1.38) | 0.11 | 1.20 (1.00 to 1.44) | 0.048 |
| IL-33 | 1.45 (1.13 to 1.87) | 0.003 | 1.45 (1.13 to 1.87) | 0.004 |
| IFN-γ | 0.87 (0.75 to 1.01) | 0.07 | 0.92 (0.79 to 1.07) | 0.29 |
| TNFα | 1.28 (0.64 to 2.55) | 0.48 | 1.51 (0.70 to 3.24) | 0.29 |
Bold indicates statistical significance at alpha level 0.05.
Adjusted for age, gender, household income, and current ETS exposure.
Discussion
To our knowledge, this is the first study to examine residential distance to a major road and a panel of plasma cytokines (enriched for the TH17 pathway) in children with and without asthma.
Among Puerto Rican children, residential proximity to a major road is significantly associated with increased plasma levels of IL-31 (implicated in TH2 immune responses). There was also suggestive, but nonstatistically significant evidence of an association between proximity to a major road and higher levels of both IL17F (implicated in TH17 immune responses) and IL-10 (involved in pathways related to Tregs, TH2, and TH17 immune responses). Moreover, we report that asthma modifies the estimated effect of proximity to a major road on plasma level of TNF-α. Whereas living further away from a major road was associated with a lower level of TNF-α in control subjects, there was no significant association between road proximity and either IL-4 or TNF-α level in children with asthma.
Our results suggest that TRAP increases plasma levels of proinflammatory cytokines, including TH2 (eg, IL-31) and TH17 (eg, IL17-F) cytokines among subjects in an ethnic group that is disproportionately affected with asthma (Puerto Ricans). These findings are generally consistent with those of a recent study in mice, which reported that exposure to urban particulate matter (PM) is associated with higher serum levels of IL-4 and IL-17F, although that study reported lower serum levels of IL-10.15
IL-31 is a relatively novel cytokine that is induced by IL-4, is produced by TH2 cells (but not TH1, TH17, or TH22), and promotes TH2-type inflammation.16 IL-31 level has been associated with multiple atopic and hypersensitivity conditions, including asthma, eczema,17 allergic rhinitis,18 mastocytosis,19 and pruritus in a variety of disorders.20–22 However, little is known about environmental factors that influence IL-31 production; to our knowledge this is the first study to show that TRAP is associated with increased IL-31 in plasma.
TNF-α has been widely implicated in asthma.23–25 In vitro or animal studies have shown that exposure to ozone, diesel exhaust particles, or near-roadway PM can induce production of TNF-α.26,27 Similar results have been found in bronchoalveolar fluid from mice exposed to high concentrations of PM2.5.4 Interestingly, we found that TNF-α decreased in children without asthma who live farther from major roadways, whereas no such decrease was seen among children with asthma. Thus, we speculate that asthma may abrogate the beneficial effect of living farther from areas with high TRAP.
Consistent with our previous finding of a predominance of atopic or allergic asthma among Puerto Rican children, plasma levels of IL-22 and IL-33 (implicated in TH2 immune responses) and IL-1β (a proinflammatory cytokine) were significantly higher in cases than in control subjects (P < 0.01 in all instances). There was also a nonstatistically significant trend for a higher level of IL-31 (implicated in TH2 immune responses) in cases than in control subjects (P < 0.05).
We recognize several limitations of our study. First, we lack data on specific pollutants, and thus cannot examine their individual effects on cytokine levels. However, examining TRAP by residential proximity to a major road integrates exposure to a complex milieu of pollutants,28 some of which (ex. nitrogen dioxide, PM and ozone) have been implicated in the pathogenesis of asthma and severe asthma exacerbations.29,30 Second, repeated cytokine measurements may offer a more complete picture of immune responses than a single measurement, because of better accounting for intrasubject variability31–33 or effects from acute exposures.31,34 Third, we had no cytokine measurements in the airways, thus limiting our assessment of potential effects of TRAP on airway inflammation.35 Fourth, we cannot establish temporality or causation in this cross-sectional study. Fifth, we had limited statistical power to detect modest to moderate associations, due to both sample size and the need to correct for multiple testing. Sixth, we lacked data on airway responsiveness. However, our definition of asthma has been extensively used in epidemiologic studies in children, in whom it is well correlated with increased airway responsiveness to methacholine. Finally, we did not use a usual Bonferroni correction, which would adjust for all 14 cytokines measured. On the other hand, our strategy to adjust for 5 noncorrelated cytokine levels is both reasonable and similar to a broadly used approach to adjust for multiple testing in the context of correlated genetic markers (eg, linkage disequilibrium-adjusted Bonferroni correction14). Such approach is less stringent, resulting in a lower probability of a Type II error (eg, falsely negative results).
In summary, our findings suggest that roadway proximity, a marker of TRAP, is associated with increased levels of several proinflammatory cytokines in Puerto Rican children. Our results warrant follow up in longitudinal studies of under-served children with and without asthma, including Puerto Ricans.
Supplementary Material
Acknowledgments
This work was supported by grants HL079966 and HL117191 from the US National Institutes of Health, and by The Heinz Endowments. Dr Forno's contribution was funded in part by grants HL125666 from the US NIH, and the Children's Hospital of Pittsburgh of UPMC. None of the funding sponsors had any role in study design, data analysis, or article preparation or approval. Dr. Celedón had full access to all of the data and takes responsibility for the integrity and accuracy of the analysis. The authors thank all participating children and their families for their invaluable contribution to the study.
Authors’ Contributions
Conception and study design: F.R., G.C., and J.C.C.; Data collection: N.B., A.C.-S., M.A., and E.A.-P; Data analysis and interpretation: F.R., E.F., J.B. Y.-Y.H., K.S.K., and J.F.A.; drafting of the article for intellectual content: F.R. and J.C.C. All authors approved the final version of the article prior to submission.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, et al. . Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012:1–8 [PubMed] [Google Scholar]
- 2.Brown MS, Sarnat SE, DeMuth KA, et al. . Residential proximity to a major roadway is associated with features of asthma control in children. PLoS One 2012; 7:e37044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura KK, Galanter JM, Roth LA, et al. . Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med 2013; 188:309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhong W, Meng Q, et al. . Ambient PM2.5 exposure exacerbates severity of allergic asthma in previously sensitized mice. J Asthma 2015; 52:785–794 [DOI] [PubMed] [Google Scholar]
- 5.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. . Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 2007; 357:2348–2358 [DOI] [PubMed] [Google Scholar]
- 6.van Voorhis M, Knopp S, Julliard W, et al. . Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One 2013; 8:e82545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt EB, Kovacic MB, Lee GB, et al. . Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol 2013; 132:1194–1204.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosser F, Brehm JM, Forno E, et al. . Proximity to a major road, vitamin D insufficiency, and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2014; 190:1190–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas-Salazar C, Ramratnam SK, Brehm JM, et al. . Prematurity, atopy, and childhood asthma in Puerto Ricans. J Allergy Clin Immunol 2014; 133:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal MN, Banks-Schlegel S, Bleecker ER, Marsh DG, Ober C. Collaborative studies on the genetics of asthma—National Heart, Lung and Blood Institute. Clin Exp Allergy 1995; 25 Suppl 2:29–32 [DOI] [PubMed] [Google Scholar]
- 11.Perez-Perdomo R, Perez-Cardona C, Disdier-Flores O, Cintron Y. Prevalence and correlates of asthma in the Puerto Rican population: behavioral risk factor surveillance system, 2000. J Asthma 2003; 40:465–474 [DOI] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. . CDC growth charts: United States. Adv Data 2000:1–27 [PubMed] [Google Scholar]
- 13.Freeman NC, Schneider D, McGarvey P. Household exposure factors, asthma, and school absenteeism in a predominantly Hispanic community. J Expo Anal Environ Epidemiol 2003; 13:169–176 [DOI] [PubMed] [Google Scholar]
- 14.McCarthy MI, Abecasis GR, Cardon LR, et al. . Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 2008; 9:356–369 [DOI] [PubMed] [Google Scholar]
- 15.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect 2010; 118:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol 2013; 132:446–454.e5 [DOI] [PubMed] [Google Scholar]
- 17.Kato A, Fujii E, Watanabe T, et al. . Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J Dermatol Sci 2014; 74:229–235 [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Luo R, Chen Y, et al. . Interleukin-31 promotes helper T cell type-2 inflammation in children with allergic rhinitis. Pediatr Res 2015; 77:20–28 [DOI] [PubMed] [Google Scholar]
- 19.Hartmann K, Wagner N, Rabenhorst A, et al. . Serum IL-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adult patients. J Allergy Clin Immunol 2013; 132:232–235 [DOI] [PubMed] [Google Scholar]
- 20.Cedeno-Laurent F, Singer EM, Wysocka M, et al. . Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clin Immunol 2015; 158:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko MJ, Peng YS, Chen HY, et al. . Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol 2014; 71:1151–1159.e1 [DOI] [PubMed] [Google Scholar]
- 22.Singer EM, Shin DB, Nattkemper LA, et al. . IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol 2013; 133:2783–2785 [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Chan-Yeung M, Becker AB, et al. . Polymorphisms of the IL-4, TNF-alpha, and Fcepsilon RIbeta genes and the risk of allergic disorders in at-risk infants. Am J Respir Crit Care Med 2000; 161:1655–1659 [DOI] [PubMed] [Google Scholar]
- 24.Al-Daghri NM, Abd-Alrahman S, Draz H, et al. . Increased IL-4 mRNA expression and poly-aromatic hydrocarbon concentrations from children with asthma. BMC Pediatr 2014; 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SD, Brown LA, Stephenson S, et al. . Characterization of a high TNF-alpha phenotype in children with moderate-to-severe asthma. J Allergy Clin Immunol 2015; 135:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Muller R, Berhane K, et al. . Inflammatory response of monocytes to ambient particles varies by highway proximity. Am J Respir Cell Mol Biol 2014; 51:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kafoury RM, Madden MC. Diesel exhaust particles induce the over expression of tumor necrosis factor-alpha (TNF-alpha) gene in alveolar macrophages and failed to induce apoptosis through activation of nuclear factor-kappaB (NF-kappaB). Int J Environ Res Public Health 2005; 2:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pratt GC, Parson K, Shinoda N, et al. . Quantifying traffic exposure. J Expo Sci Environ Epidemiol 2014; 24:290–296 [DOI] [PubMed] [Google Scholar]
- 29.Salam MT, Islam T, Gilliland FD. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Curr Opin Pulm Med 2008; 14:3–8 [DOI] [PubMed] [Google Scholar]
- 30.Auerbach A, Hernandez ML. The effect of environmental oxidative stress on airway inflammation. Curr Opin Allergy Clin Immunol 2012; 12:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J 2001; 17:909–915 [DOI] [PubMed] [Google Scholar]
- 32.Sarinho ES, Azoubel-Antunes A, Rego MJ, et al. . Evaluation of Th17-related cytokines and IFNgamma production from blood mononuclear cells of moderate and severe asthmatic children reveals methylprednisolone does not decrease IL-22 levels. J Asthma 2015; 52:227–231 [DOI] [PubMed] [Google Scholar]
- 33.Pukelsheim K, Stoeger T, Kutschke D, Ganguly K, Wjst M. Cytokine profiles in asthma families depend on age and phenotype. PLoS One 2010; 5:e14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behndig AF, Larsson N, Brown JL, et al. . Proinflammatory doses of diesel exhaust in healthy subjects fail to elicit equivalent or augmented airway inflammation in subjects with asthma. Thorax 2011; 66:12–19 [DOI] [PubMed] [Google Scholar]
- 35.Hollander C, Sitkauskiene B, Sakalauskas R, Westin U, Janciauskiene SM. Serum and bronchial lavage fluid concentrations of IL-8, SLPI, sCD14 and sICAM-1 in patients with COPD and asthma. Respir Med 2007; 101:1947–1953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.