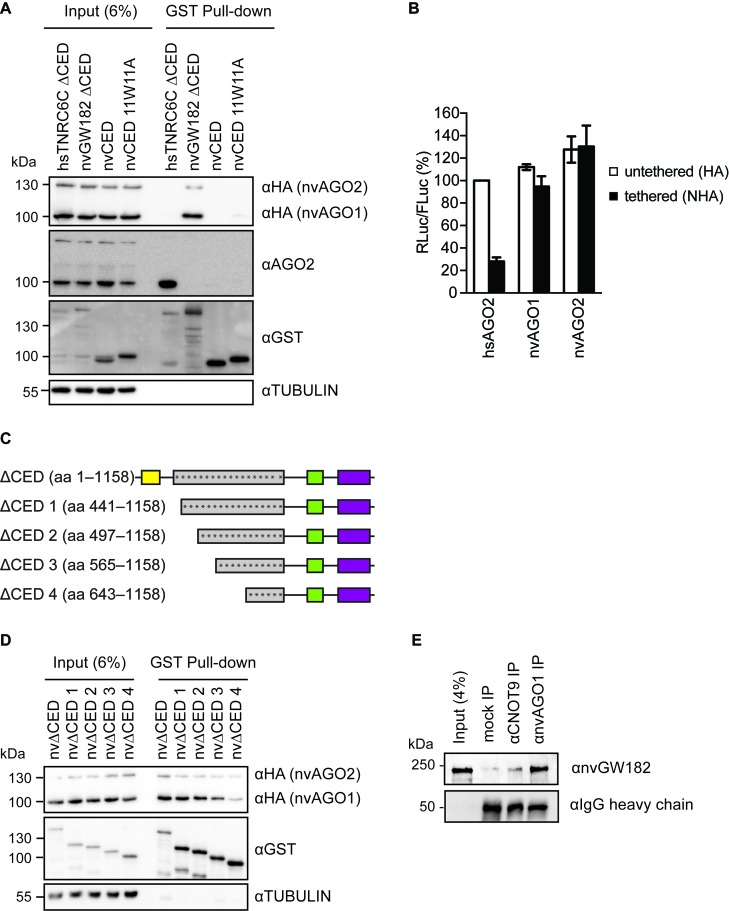
Figure 5.
GW182-AGO interaction regions are not conserved between human and Nematostella. (A) nvGW182 binds nvAGOs, but not human AGO2. GST-fusions of nvGW182 or its domains were co-expressed with HA-tagged nvAGO1 and nvAGO2 in HEK293 cells, pulled down with GST tag, and inputs (6%) and GST-pulldowns were analyzed by Western blotting using the indicated antibodies. Human TNRC6CΔCED was used as positive control for human AGO2 binding. (B) Nematostella AGOs are not able to repress tethered mRNA in human cells. Human HEK293 cells were co-transfected with RLuc-boxB, FLuc and NHA-nvAGO1 or NHA-nvAGO2. Human AGO2 (NHA-hsAGO2) was used as a positive control. As negative controls, plasmids encoding untethered HA-fusions were used (means ±SD, n = 3). (C) Schematic representation of nvΔCED deletion mutants used in (D). The numbers correspond to the amino acid positions. Asterisks represent putative AGO-binding motifs. (D) Multiple regions of nvΔCED contribute to nvAGOs binding. GST-fusions of nvΔCED deletion mutants (C) were co-expressed with HA-nvAGOs in HEK293 cells, and inputs (6%) and GST-pulldowns were analyzed by Western blotting using the indicated antibodies. (D) AGO, GW182 and the CCR4-NOT complex natively interact in Nematostella. Nematostella embryonic lysates were used in IP with αnvAGO1 and αCNOT9 antibody, and the co-immunoprecipitated proteins were analyzed by Western blotting with αnvGW182 antibody. Unspecific rabbit IgG was used as a negative control.