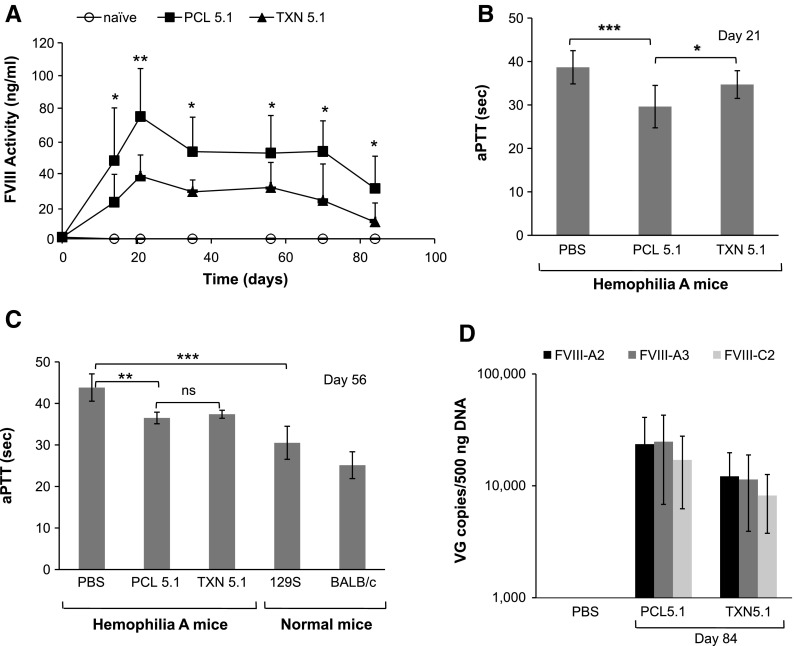
Figure 5.
Comparison of PCL- and TXN-produced AAVrh8R/FVIII-5.1 vectors in vivo using hemophilia A mice. Vectors were administered via the tail vein at 4 × 1010 vg/mouse. (A) Plasma FVIII protein activity. Activity was measured in day 21, 35, 56, 70, and 84 samples using the Coatest® assay. (B) Plasma clotting times on day 21 measured by the aPTT assay. (C) Plasma clotting times on day 56. The clotting times for mouse strains 129S and BALB/c are shown for comparison. (D) Vector genome levels in the liver. Vector genome copies on day 84 were quantitated by qPCR using three primer/probe sets (FVIII-A2, FVIII-A3, and FVIII-C2). For all analyses, the method of virus production is indicated (PCL or TXN). All treatment groups contained eight mice per group. Values in each graph represent the average ± standard deviation, and significance was calculated using Student's t-test, with significance indicated as *p < 0.05, **p < 0.01, and ***p < 0.001.